Alternative methods to animal toxicity testing are needed because animal testing can cause suffering. Some alternative methods include computer modeling, cell and tissue cultures, and organ chips. Computer modeling uses computer programs to simulate biological effects and predict toxicity without animal testing. Cell and tissue cultures grow isolated cells and tissues in vitro to study toxicity. Organ chips are microfluidic devices that contain living cells arranged to simulate organ-level functions, allowing tests on human-relevant systems without whole animals. These alternative methods can help reduce and replace animal use in toxicity testing.






![Alternative to Animal Experiments
Continued but
modified use of
animals 5Rs
In vitro [test tube]
method
Tissue culture
technique
In silico
[computer
modelling
technique]
Computer aided molecular drug design [CADD]
Computer assisted learning [CAL]
Microfluidic chips
Quantitative structure activity relationship
Organ on chips
Computer or mathematic analysis
In silico
[computer
modelling
technique] 6](https://image.slidesharecdn.com/alternativemethodsofanimaltoxicity-230724143735-0da52aad/75/alternative-methods-of-animal-toxicity-pptx-8-2048.jpg)

![Methods Of Refinement
Providing relief [pain and distress] by giving drugs like analgesics,
anesthetics, tranquillizers and sedatives.
By changing procedure
Modified to
reduce pain and
distress in
animal
Use non
invasive
technique like
MRI
Use less
sensitive animal
species
Use smaller
dose
Improving
housing
conditions
8](https://image.slidesharecdn.com/alternativemethodsofanimaltoxicity-230724143735-0da52aad/75/alternative-methods-of-animal-toxicity-pptx-10-2048.jpg)

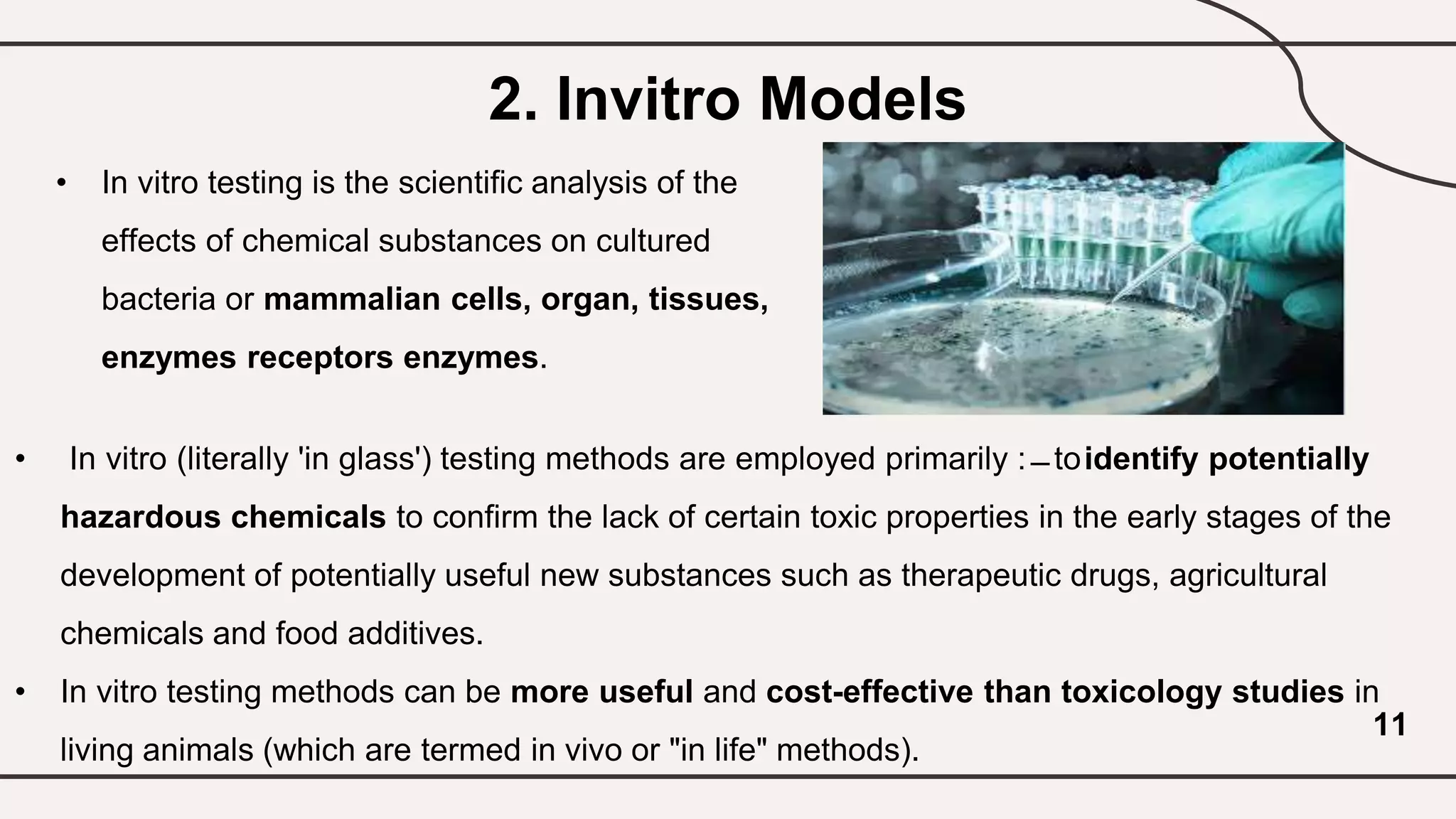
![Various Test In Vitro Methods
● In vitro pyrogen test
● Embryonic stem cell test
● Carcinogenicity test
● Neurotoxicity test
Avian chick embryo
Rodents[rat and mice, wild
type, transgenic , embryonic,
post natal , adult
Human cells [neural
progenitor cells from
aborted fetus and stem
cell lines]
Source Of Tissue For In Vitro Methods
12](https://image.slidesharecdn.com/alternativemethodsofanimaltoxicity-230724143735-0da52aad/75/alternative-methods-of-animal-toxicity-pptx-14-2048.jpg)

![4. In silico [Computer Modelling] Techniques
● Without animal dissection computer generated stimulation are used to predict the
various possible biological and toxic effects of a chemical or potential drug candidate.
● VARIOUS TYPES IN SILICO MODELS
Computer aided molecular drug design [CADD]
Quantitative structure activity relationship
Computer assisted learning[CAL]
Computer or mathematical analysis
Organ on chips 14](https://image.slidesharecdn.com/alternativemethodsofanimaltoxicity-230724143735-0da52aad/75/alternative-methods-of-animal-toxicity-pptx-16-2048.jpg)
![A. Computer Aided Drug Design [CADD]
● It is the inventive process of finding new medications based
on the knowledge of a biological target.
● It involves the design of molecules that are complementary in
shape and charge to the biomolecular target with which they
interact and therefore will bind to it.
● It is used to predict the receptor binding site for a potential drug
molecule.
● CADD works to identify the probable binding site and hence
avoid testing of unwanted chemicals having no biological
activity. Computational approach to discover, develop and
analyze drugs.
15](https://image.slidesharecdn.com/alternativemethodsofanimaltoxicity-230724143735-0da52aad/75/alternative-methods-of-animal-toxicity-pptx-17-2048.jpg)







