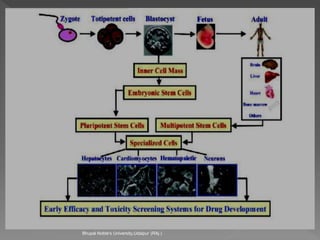
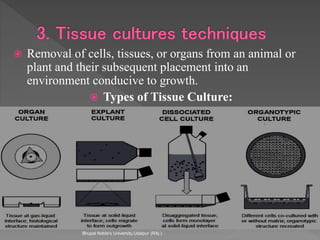
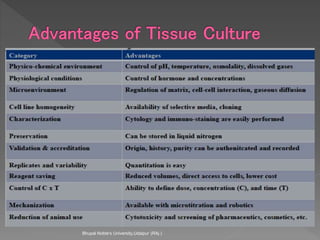
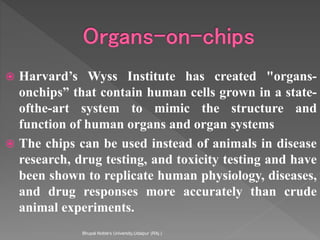
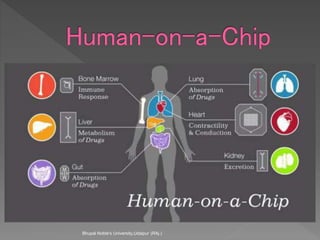
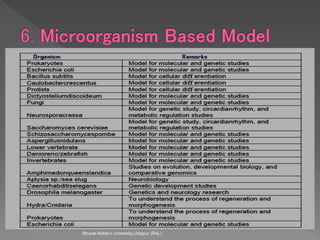
Animals are used in various areas of biomedical science such as teaching, research, and testing of drugs. While animal models provide important insights, they have limitations in translating findings to humans due to interspecies differences. To reduce animal use, alternatives such as computer modeling, tissue cultures, and microdosing are being utilized. The 3Rs principle of replacement, reduction, and refinement is also applied to minimize animal pain and distress when animal use is necessary.