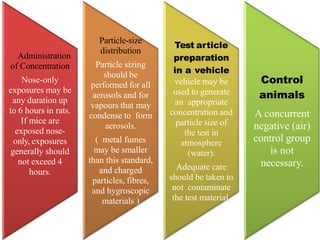
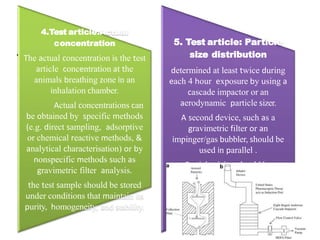
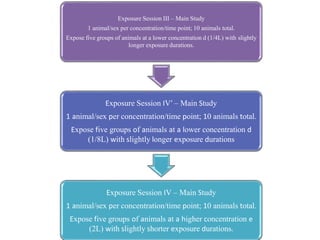
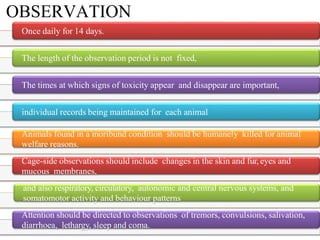
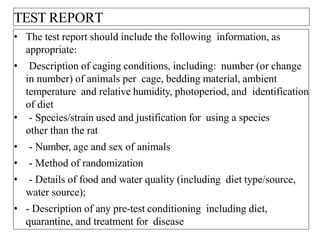
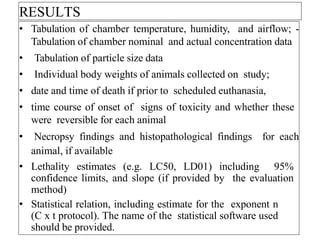
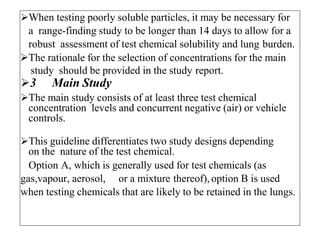
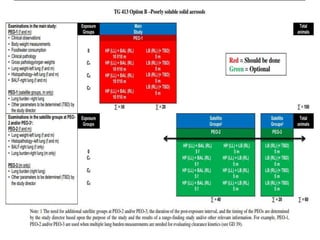
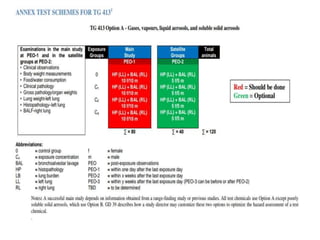
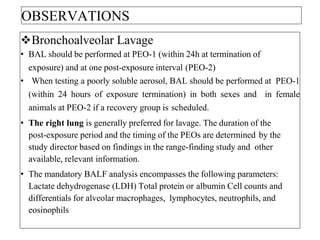
This document summarizes guidelines for inhalation toxicity studies including acute, subacute, and subchronic toxicity studies. It describes the objectives, principles, procedures, observations, and reporting requirements for these studies. Key aspects covered include selecting animal species and exposure concentrations, monitoring exposure conditions, conducting traditional and CxT exposure protocols, observing animals for signs of toxicity, and reporting study results including toxicity estimates. The purpose of these studies is to evaluate the toxic effects of inhaled substances over different exposure durations.