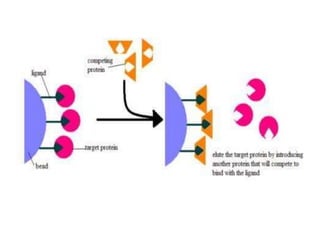
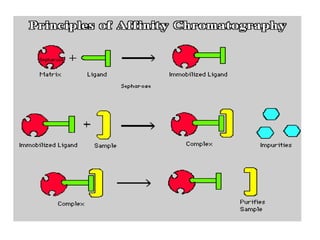
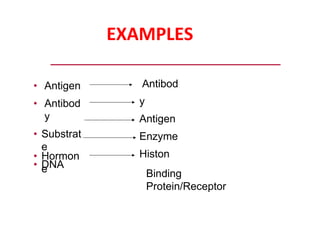
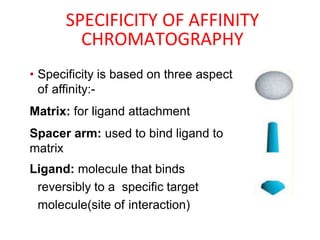
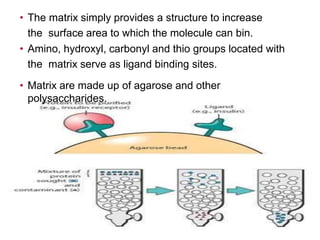
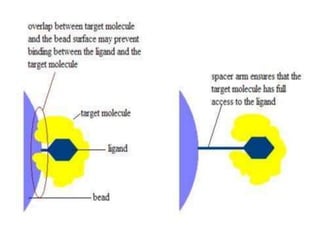
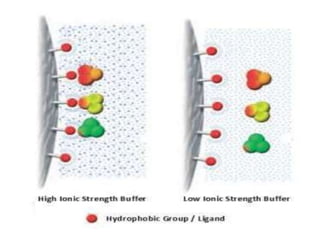
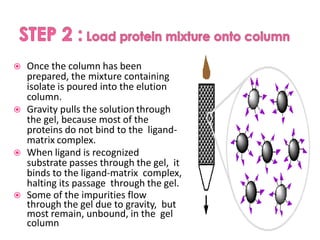
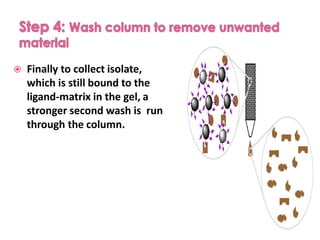
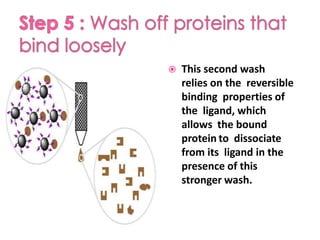
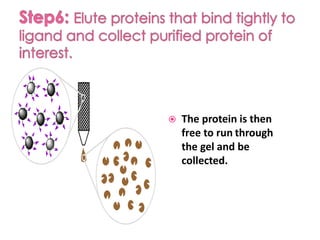
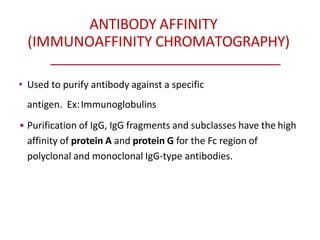
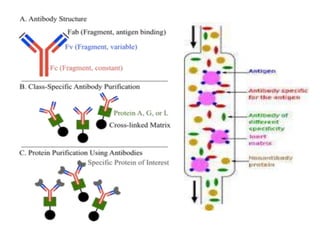
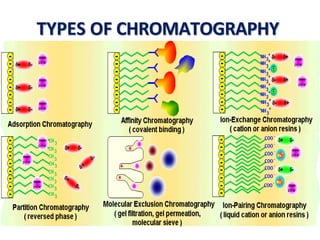
Affinity chromatography is a method used to separate biomolecules from a mixture based on specific interactions between the molecules and a ligand attached to a chromatography matrix. It was developed in the 1930s and relies on reversible interactions like enzyme-substrate or antibody-antigen binding. The target molecule binds to the ligand while unbound molecules are washed away. Then, conditions are altered to dissociate the bound molecule from the ligand, eluting it from the column in a purified form. Affinity chromatography is widely used to purify proteins, nucleic acids, and other biomolecules.
















![REFERENCES
[1]http://en.wikipedia.org/wiki/Affinity_chromatography
[2]www.apsu.edu/reedr/.../Affinity%20Chromatography%2
01.ppt [3] www.rpi.edu/dept/chem-
eng/WWW/faculty/.../Lecture%2001.pdf
[4]www.chemistryinnovation.co.uk/.../Technology%20Area
%20Affinity
%20Chromatography.pdf -](https://image.slidesharecdn.com/affinitychromatography-230114044354-aa324983/85/Affinity-Chromatography-pptx-38-320.jpg)