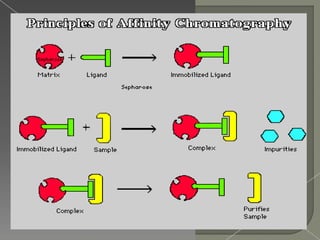
Affinity chromatography was first developed in the 1930s by Swedish biochemist Tiselius and involves using the affinity of biochemical compounds for specific properties to study enzymes and proteins. It works by having a ligand attached to an inert matrix within a column that selectively binds the desired molecule from a sample as it passes through. The molecule is then eluted from the column by changing conditions like pH or salt concentration. Affinity chromatography is widely used for purification, isolation, and research due to its high specificity and ability to obtain high purity products.