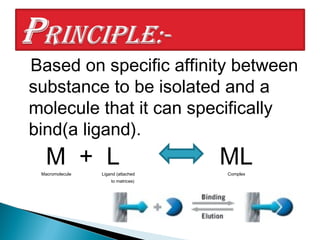
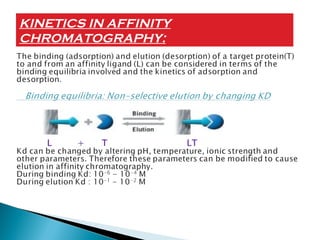
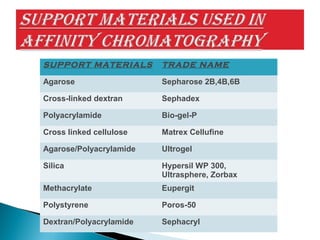
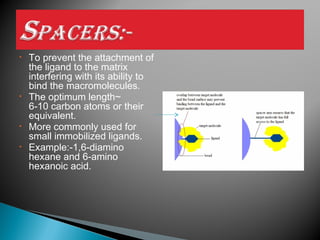
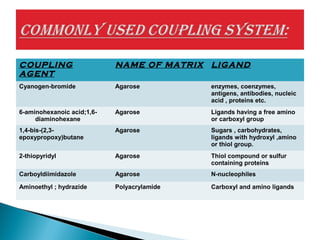
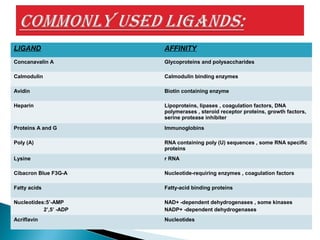
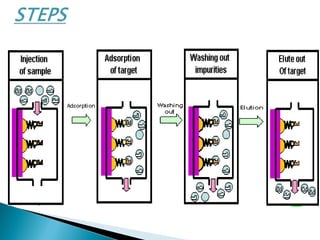
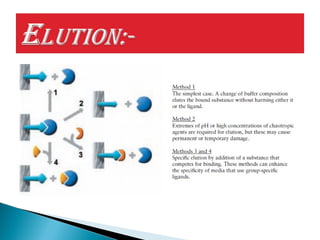
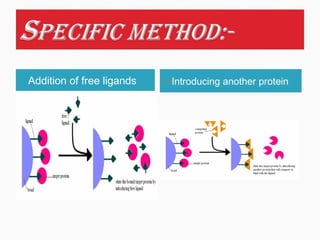
Affinity chromatography is a specialized technique within chromatography that utilizes specific binding interactions between a target substance and a ligand for the purpose of separation and purification. Key components include a chemically inert matrix for ligand attachment, various types of ligands for different target molecules, and specific methods for achieving high selectivity and yield in purification. Despite its advantages, challenges such as non-specific adsorption and high costs of immobilized ligands exist.