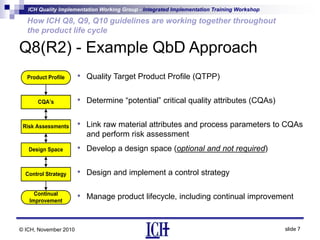
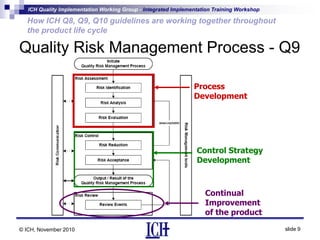
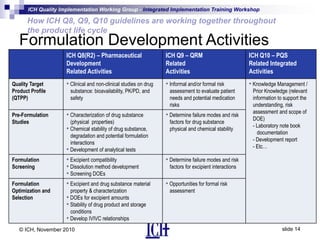
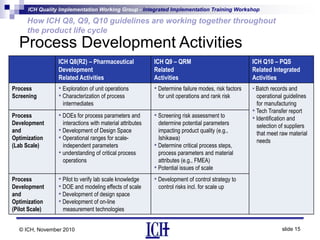
ICH Q8, Q9, and Q10 guidelines provide a risk-based and scientific approach to pharmaceutical development and manufacturing. Q8 describes pharmaceutical development and quality by design. Q9 covers quality risk management principles. Q10 addresses pharmaceutical quality systems. The guidelines work together throughout the product lifecycle from development to commercial manufacturing and continual improvement. Their integrated implementation enhances product quality and manufacturing efficiency.