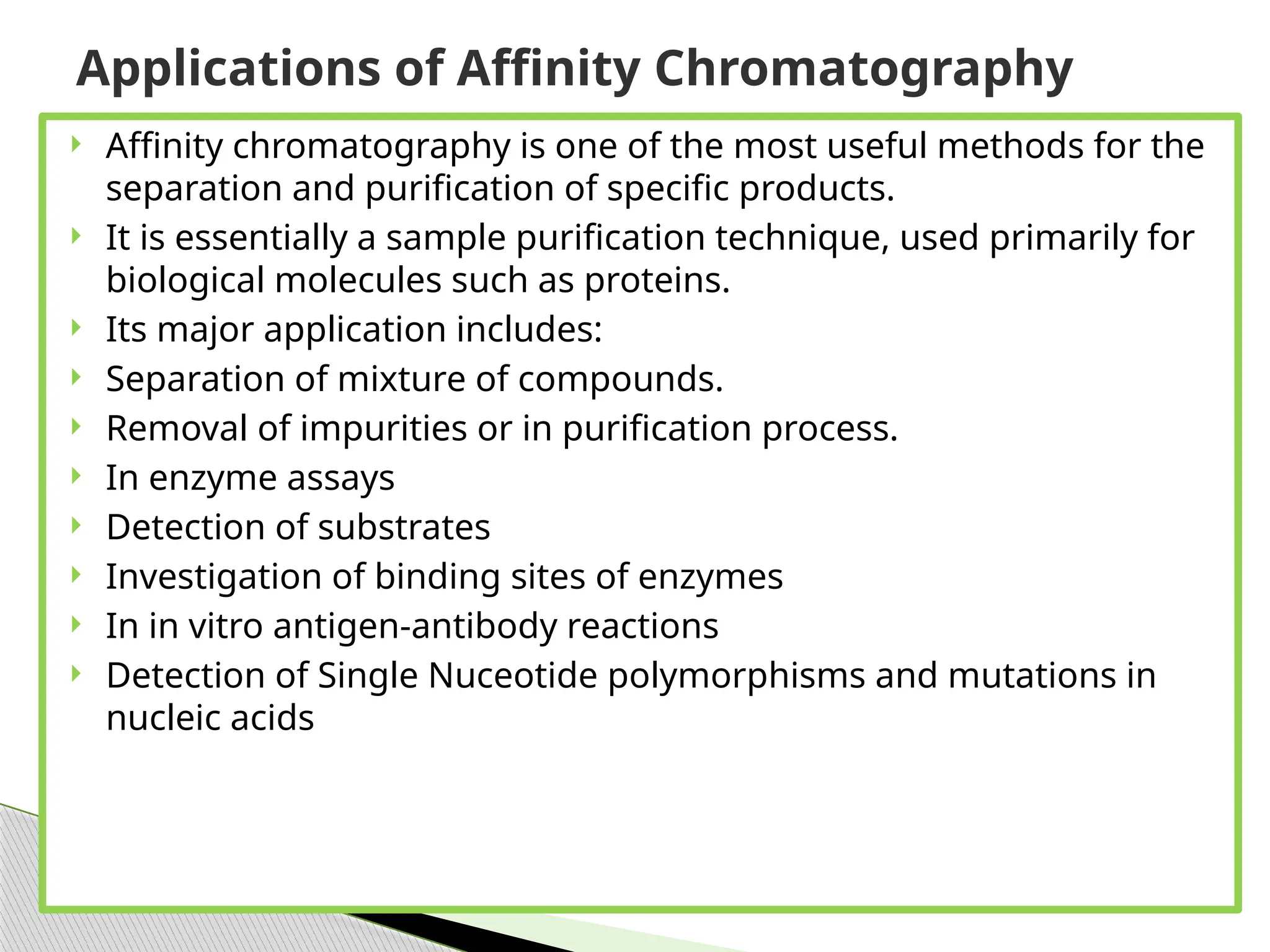
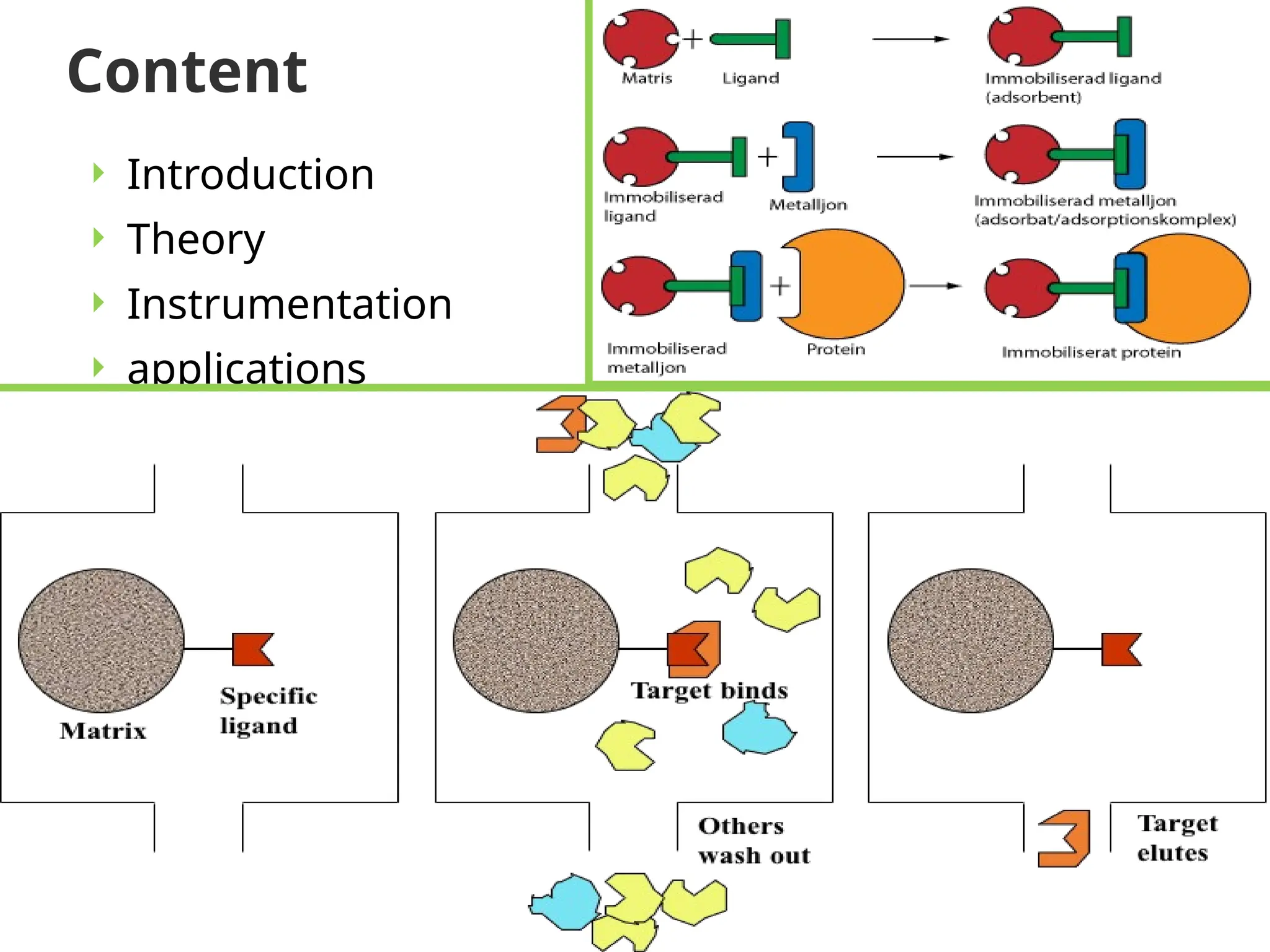
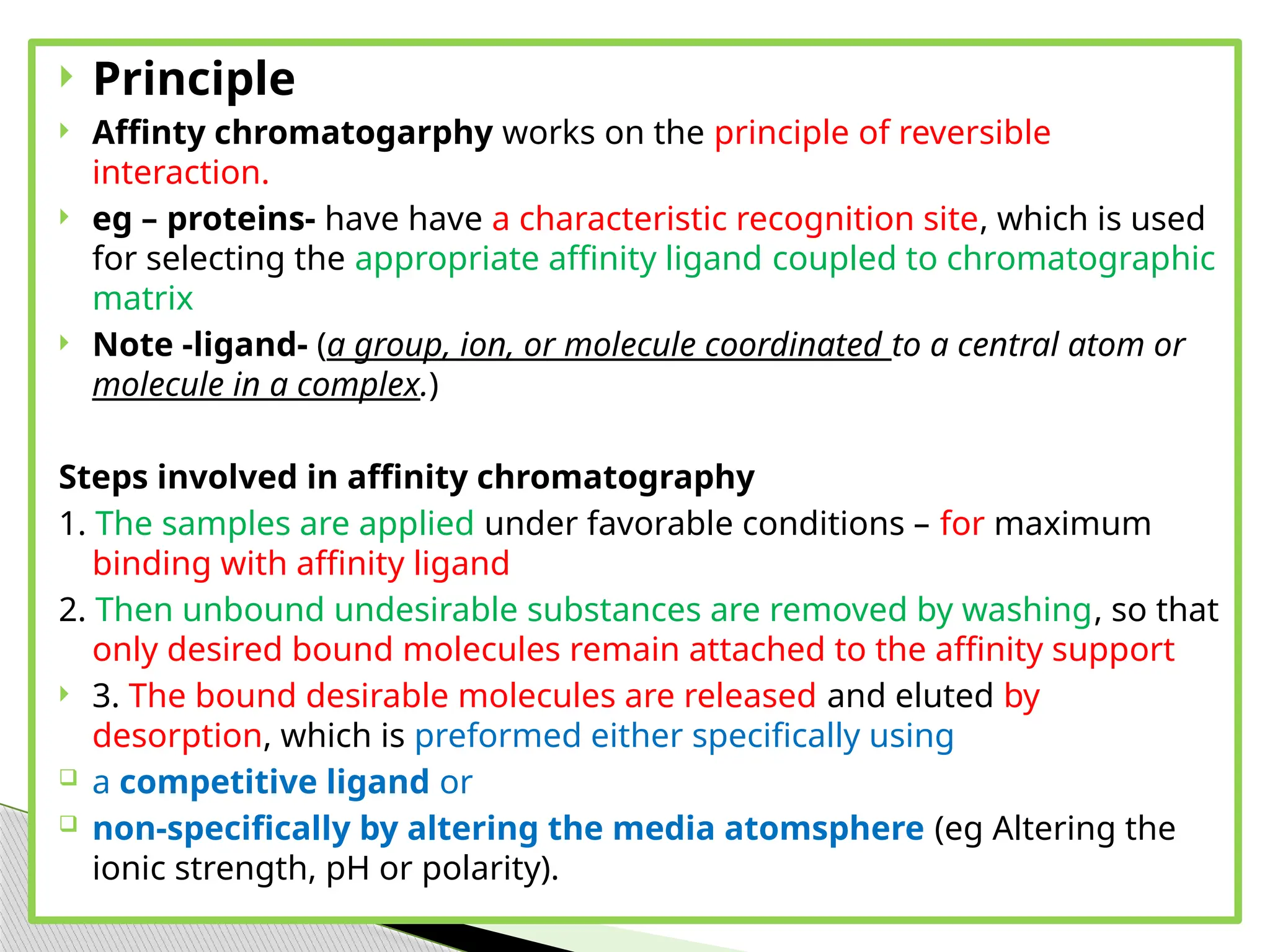
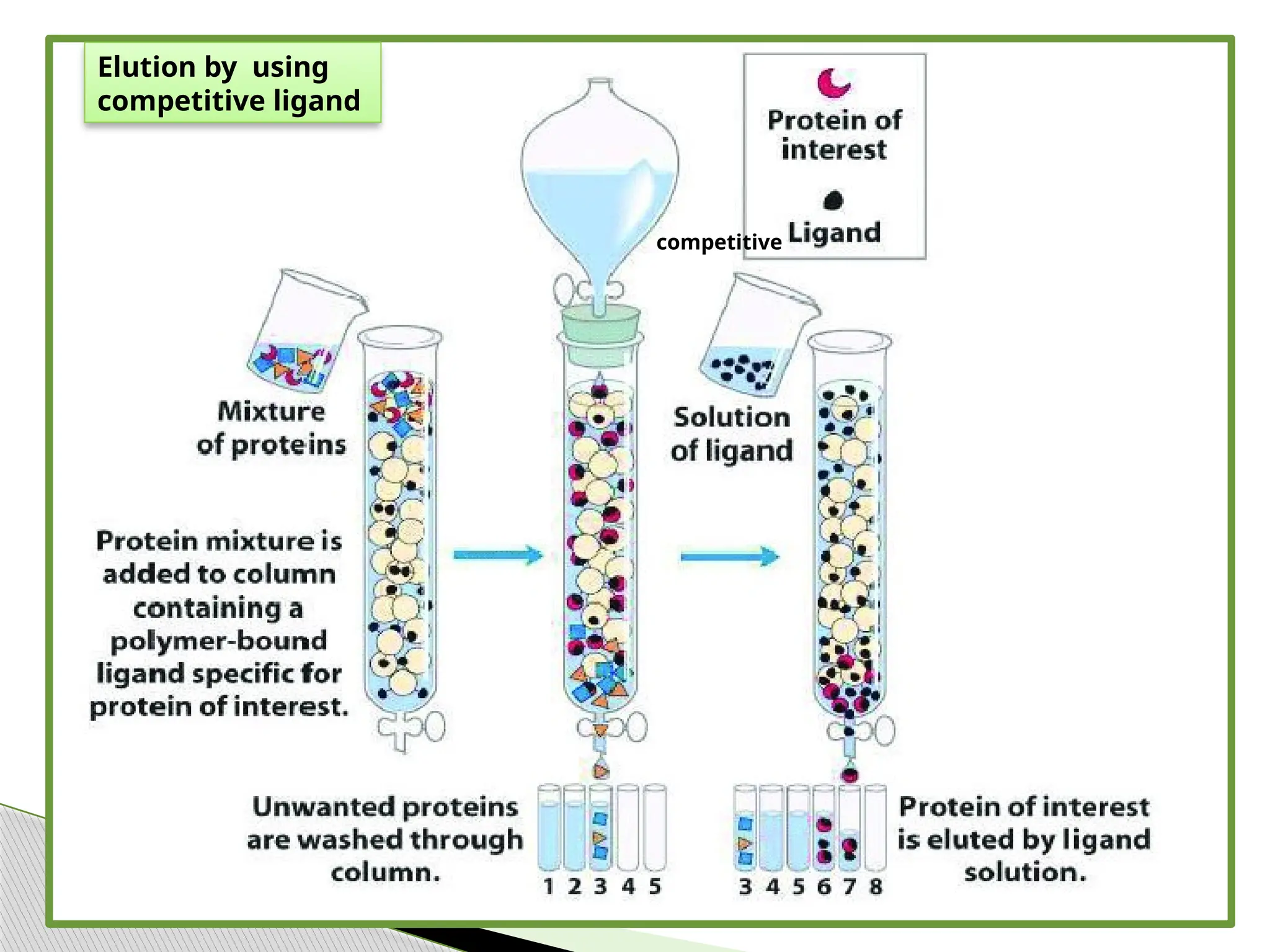
This document provides an in-depth overview of affinity chromatography, explaining its principles, steps, and applications in the separation and purification of biomolecules. It discusses how ligands are used to selectively bind to target molecules and the importance of matrix and spacer arms in the process. Additionally, the document outlines the advantages and limitations of the method, while detailing different types of affinity chromatography based on the ligands used.









![ Where, A= targeted molecule
B= Ligand
AB= Complex formed between A and B
Kd =
[A][B]
[AB]
Note-
A nucleotide building blocks, of DNA and RNA. A nucleotide consists of
a base (one of four chemicals: adenine, thymine, guanine, and cytosine) +
a molecule of sugar + one of phosphoric acid.
Nucleic acids- either of two acids (DNA and RNA), that are present in all
living cells
Immunoglobuline- any of a class of proteins present in the serum and
cells of the immune system, which function as antibodies.
An antibody, also known as an immunoglobulin, is a large, Y-shaped
protein used by the immune system to identify and neutralize foreign
objects such as pathogenic bacteria and viruses. The antibody recognizes a
unique molecule of the pathogen, called an antigen.](https://image.slidesharecdn.com/affinitychromatography1-241017125354-2c752862/75/Affinity-chromatography-Introduction-Theory-Instrumentation-applications-pptx-13-2048.jpg)