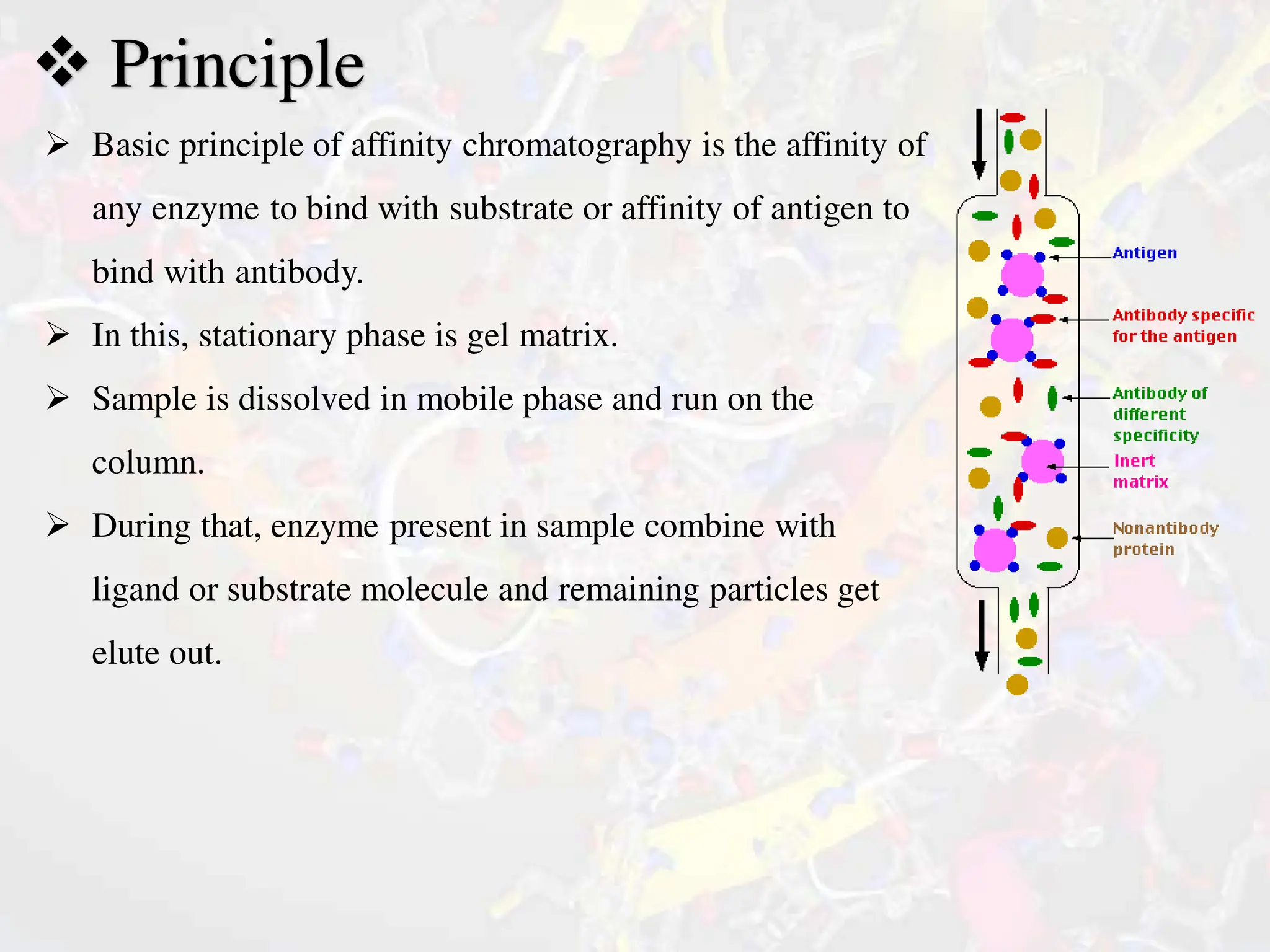
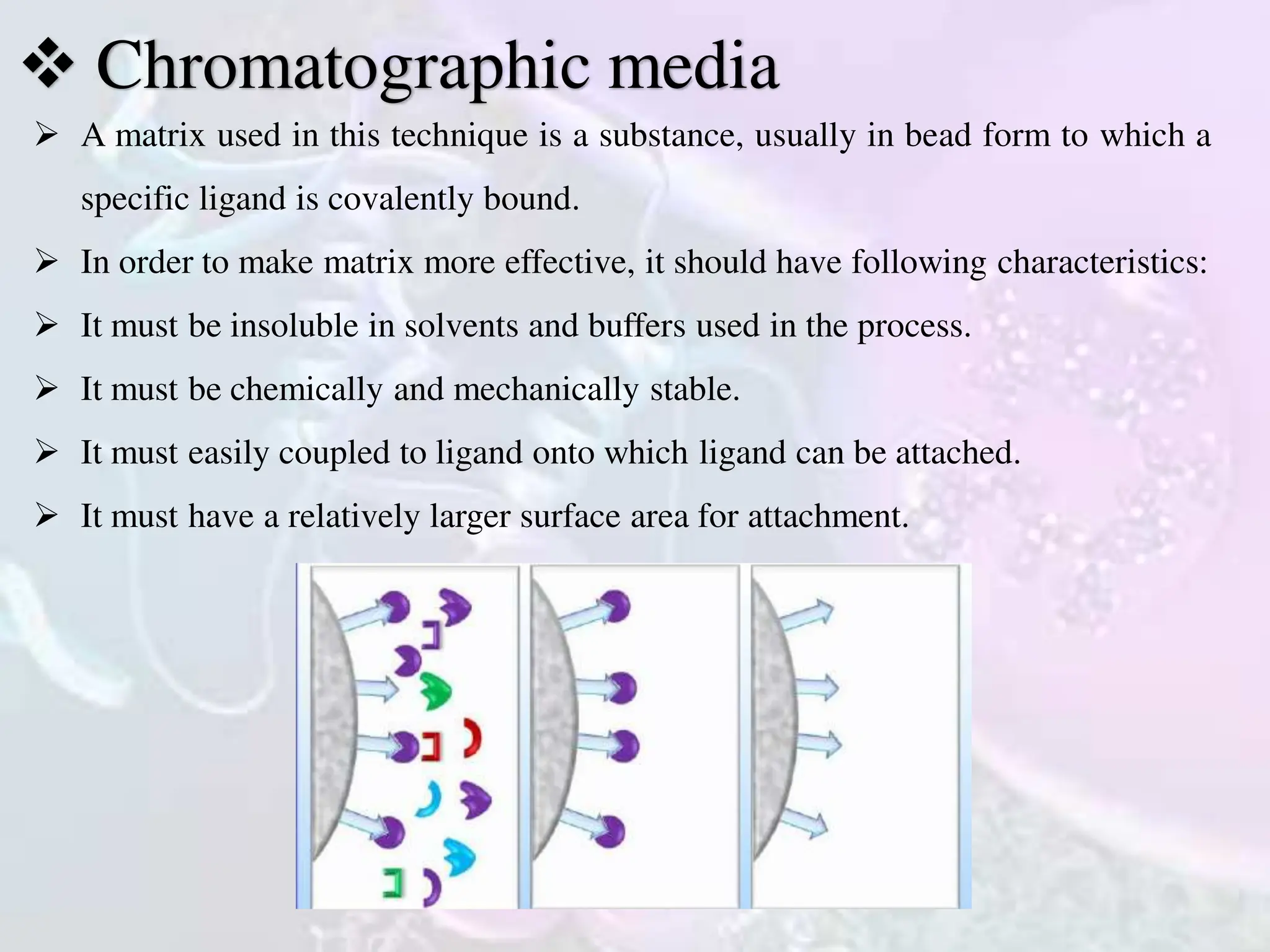
The document discusses affinity chromatography, a technique for purifying biomolecules based on specific interactions between enzymes and substrates or antigens and antibodies. It outlines the basic principles, methodology, and characteristics of chromatographic media, including the importance of selecting appropriate ligands. Applications include purifying biological macromolecules, such as nucleic acids, antibodies, and enzymes, and its relevance in genetic engineering and vaccine production.