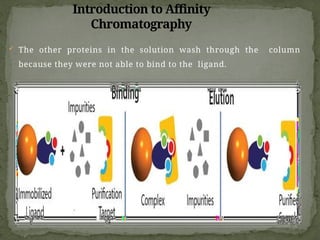
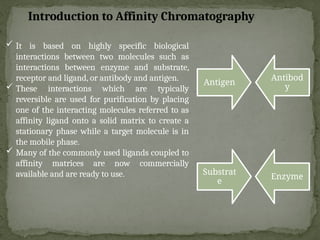
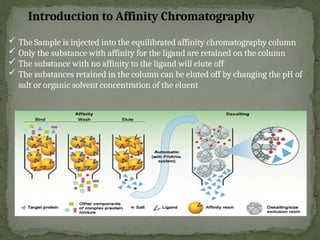
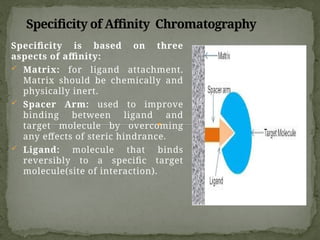
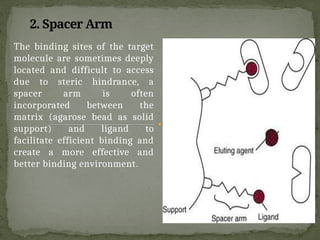
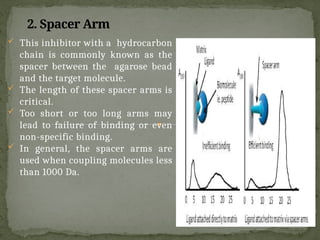
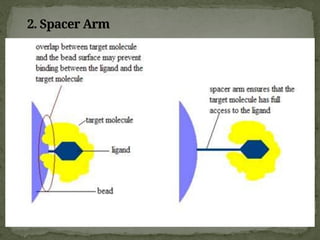
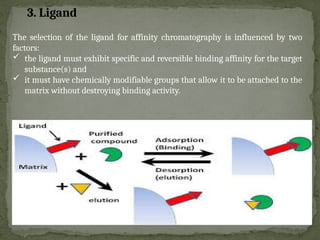
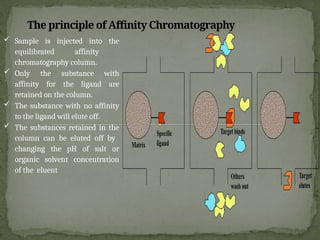
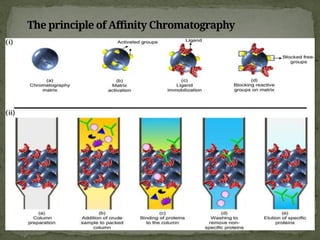
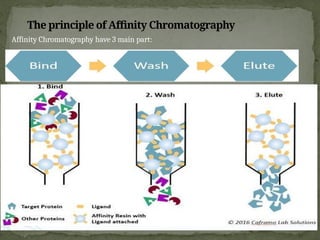
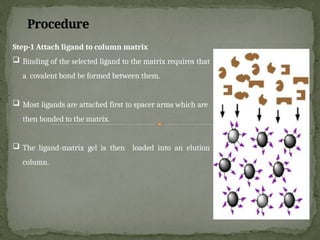
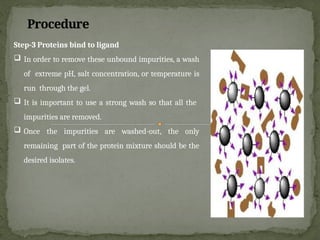
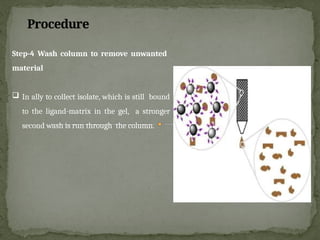
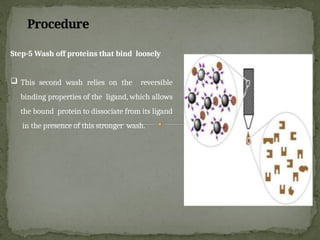
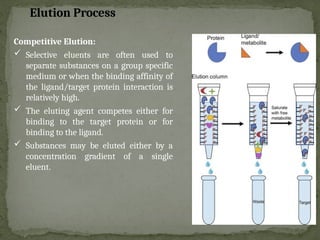
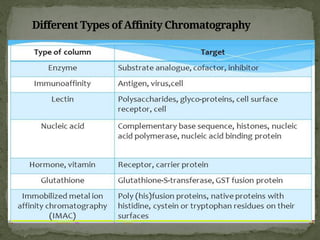
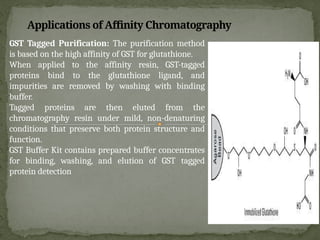
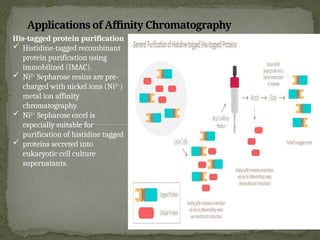
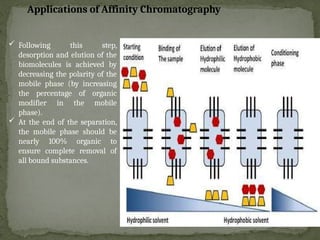
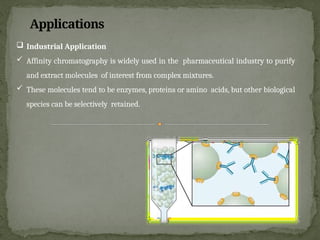
Affinity chromatography is a powerful technique developed in the 1930s for the purification of biomolecules based on specific interactions between target molecules and ligands attached to a solid support. It involves various components such as matrices and spacer arms that enhance binding efficiency, along with the elucidation of methodologies for eluting bound proteins. Applications include the purification of antibodies, tagged proteins, and immunoglobulins using different ligands and methods.