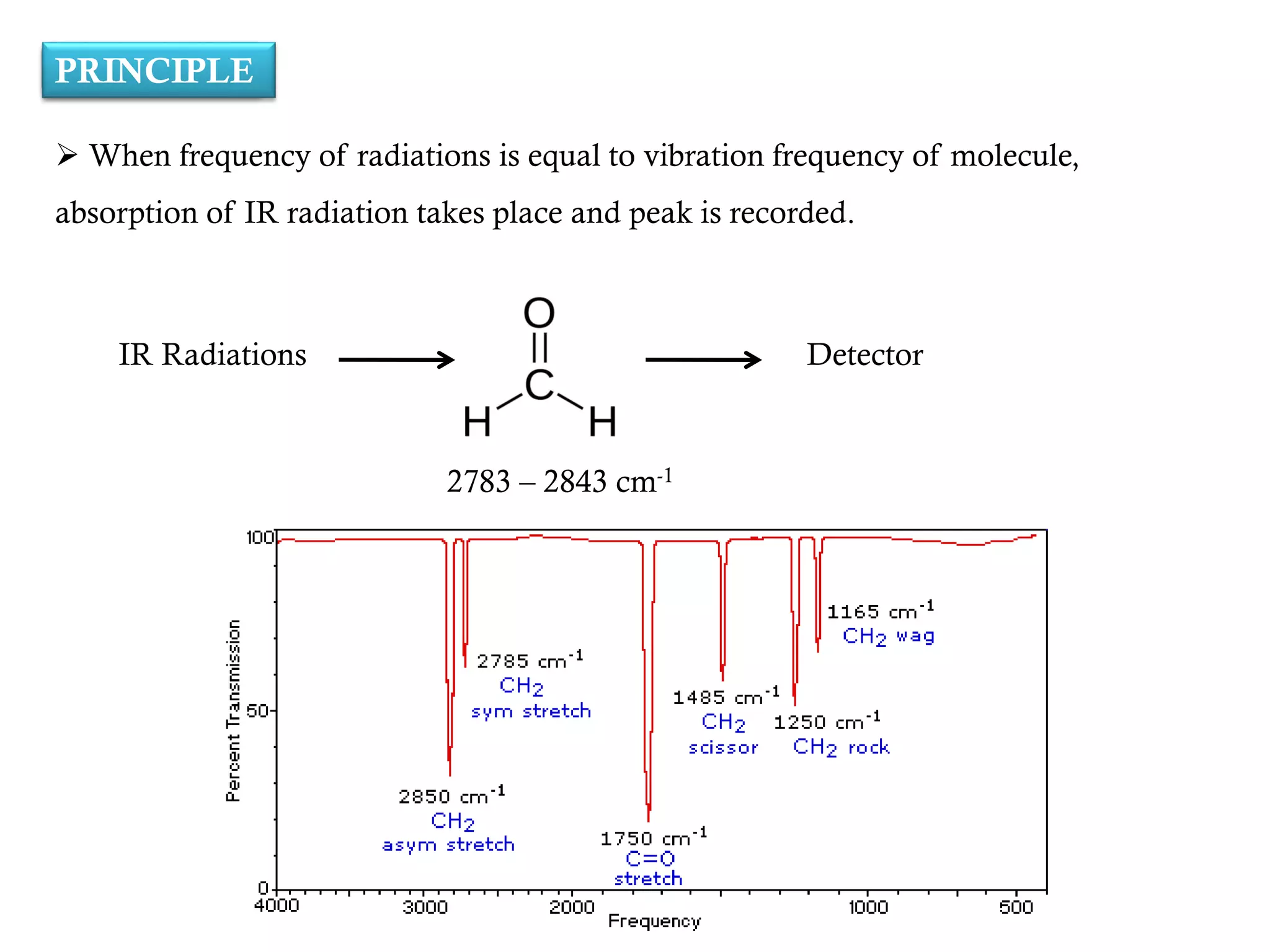
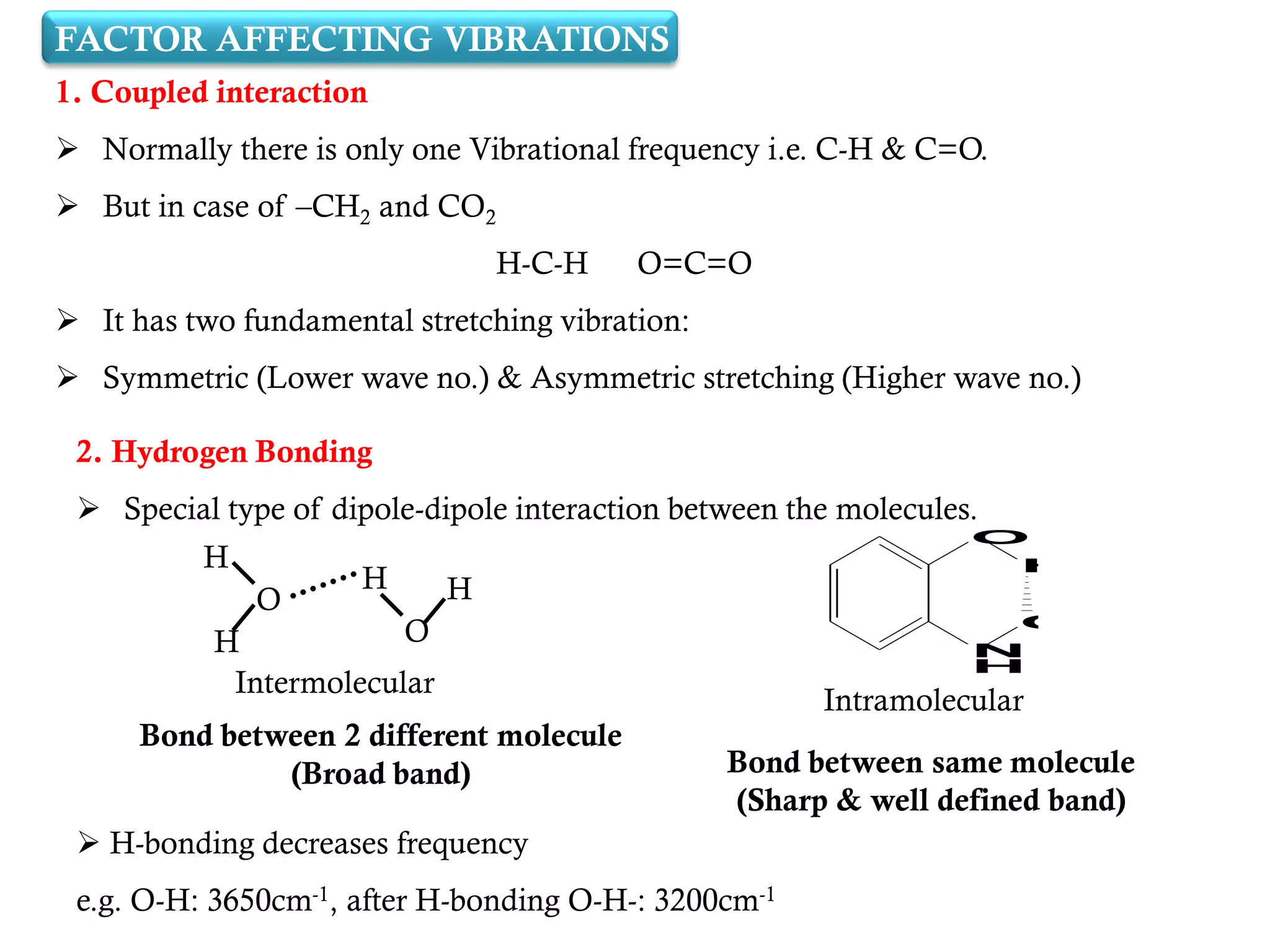
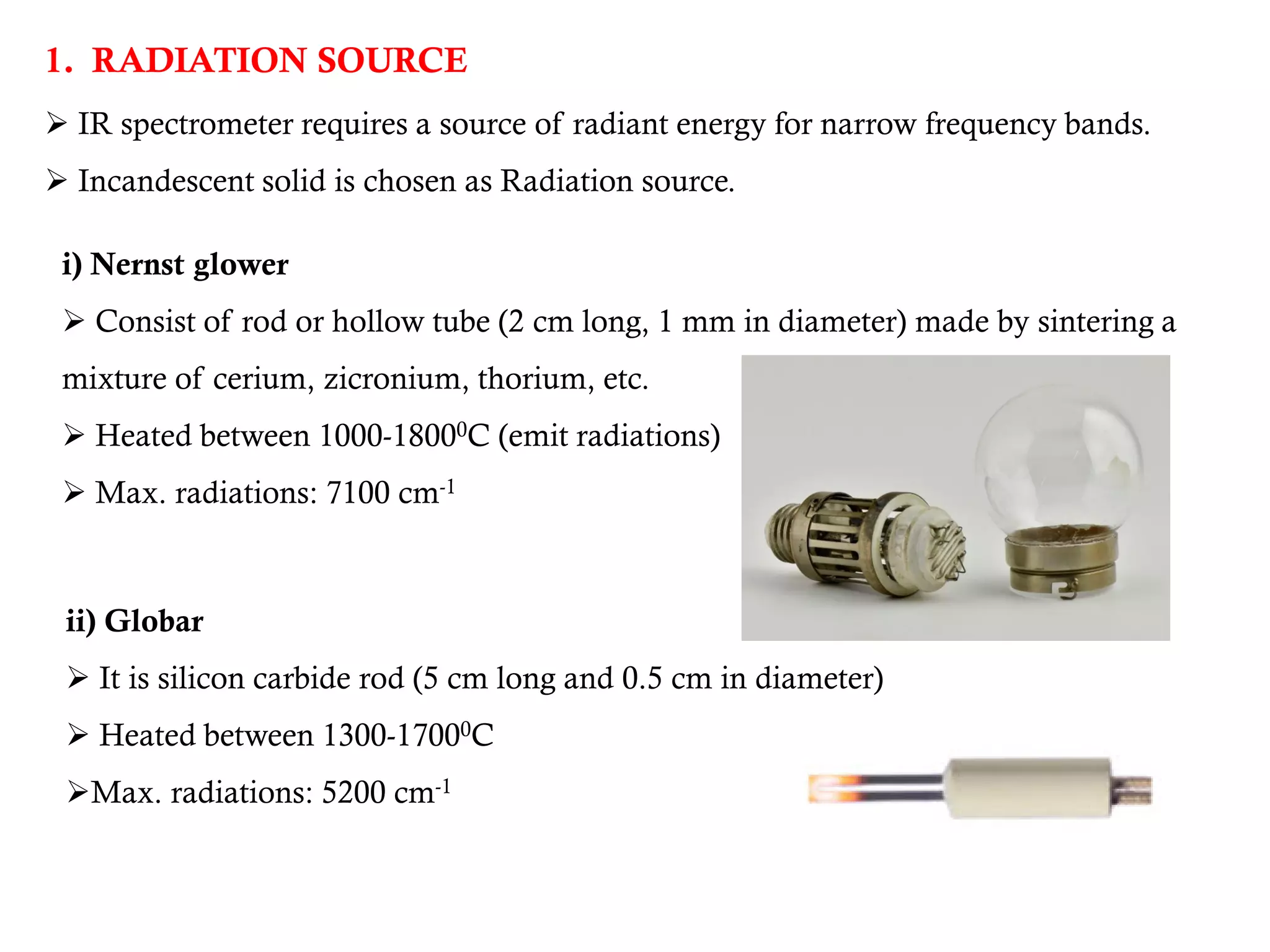
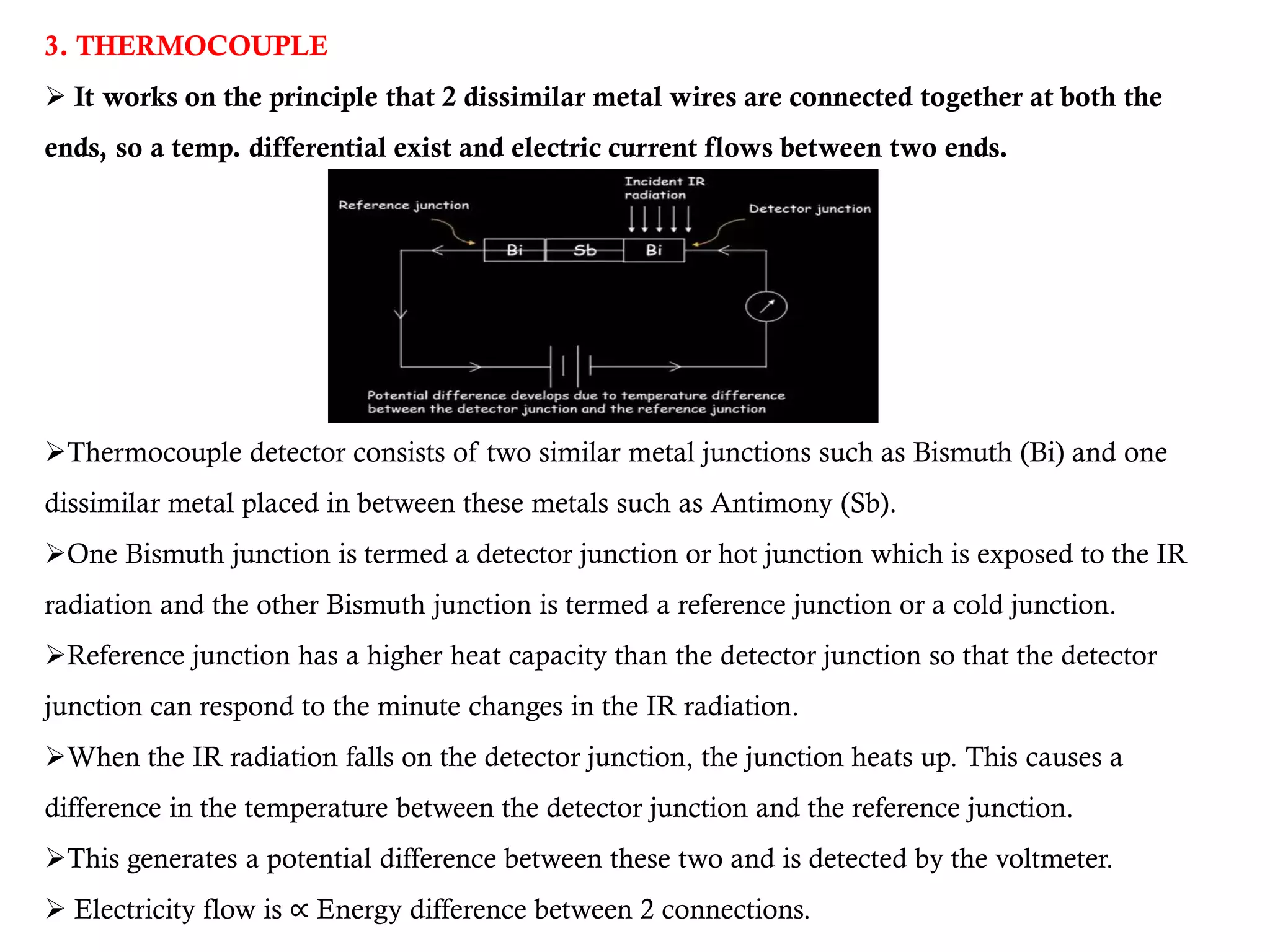
The document covers infrared (IR) spectroscopy as an analytical technique used for qualitative and quantitative analysis of samples based on their absorption of IR radiation. It details fundamental modes of molecular vibration, factors affecting these vibrations, sample handling techniques, and the instrumentation involved in IR spectroscopy. Additionally, it discusses the applications of IR spectroscopy in identifying compounds, determining structures, and studying chemical reactions.