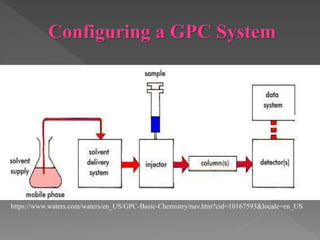
Gel chromatography, also known as size exclusion chromatography, is a technique used to separate components based on their molecular weight or size using a porous stationary phase. The method has advantages like short analysis time and defined separation, but it also has limitations such as restricted peak resolution and the necessity for filtration. Various types of gels, mobile phases, and detectors are utilized in the process, and it has applications in protein purification and molecular weight determination.