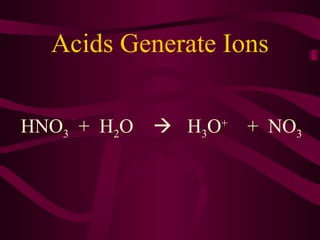
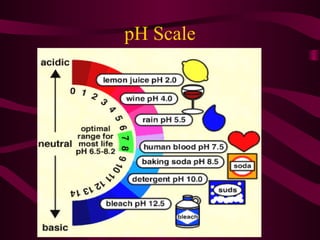
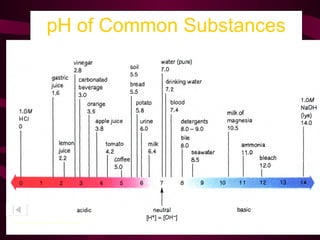
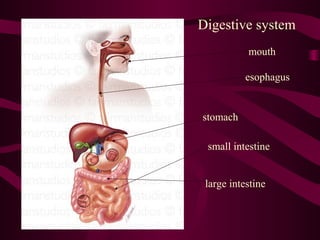
This document provides information about acids, bases, and salts. It defines acids as having a pH less than 7 and describes their properties such as neutralizing bases and forming hydrogen ions in solution. Strong acids like hydrochloric acid are described as ionizing completely, while weak acids only partially ionize. Common acids are also listed such as hydrochloric acid found in stomachs. Bases are defined as having a pH greater than 7 and their properties are outlined. The document further discusses pH scales, indicators, buffers, and examples of acid-base reactions. Digestion and pH levels in different parts of the digestive system are briefly covered.