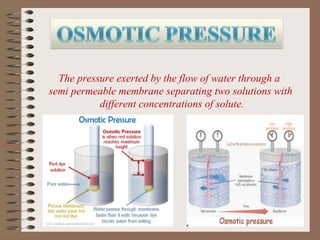
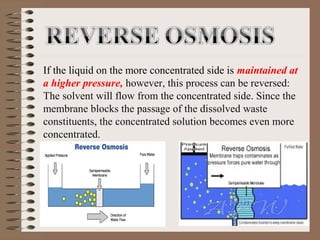
Osmosis is the movement of solvent molecules through a semi-permeable membrane from an area of higher solvent concentration to lower solvent concentration. The document defines hypertonic, isotonic, and hypotonic solutions and explains how osmosis causes cell shrinkage or swelling depending on the solution. It also provides examples of how osmosis is used in desalination, food concentration, and dairy concentration processes.