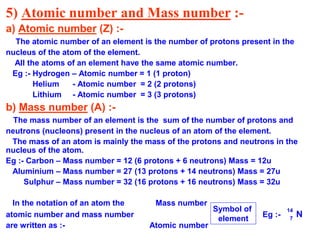
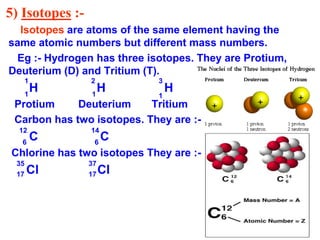
This document provides a summary of the structure of the atom. It discusses the three main subatomic particles - electrons, protons, and neutrons. It then describes the historical models of the atom including Thomson's plum pudding model, Rutherford's nuclear model, and Bohr's orbital model. Key topics covered include the distribution of electrons in shells, atomic number, mass number, isotopes, isobars, and valency.