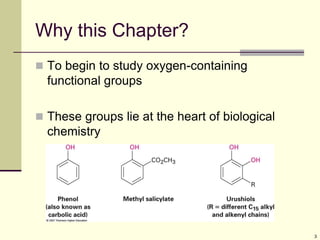
This document provides an overview of alcohols and phenols. It discusses their structures, properties, nomenclature rules, methods of preparation including reduction of carbonyl compounds and reactions of Grignard reagents, and common reactions such as oxidation, protection, and dehydration. Phenols are introduced as being more acidic than alcohols due to resonance stabilization of the phenoxide ion. Industrial production of phenol from cumene is also mentioned.







![10
Acidity Measurements
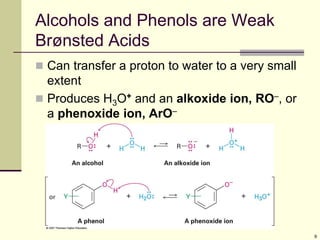
The acidity constant, Ka, measures the extent to
which a Brønsted acid transfers a proton to water
[A] [H3O+]
Ka = ————— and pKa = log Ka
[HA]
Relative acidities are more conveniently presented on
a logarithmic scale, pKa, which is directly proportional
to the free energy of the equilibrium
Differences in pKa correspond to differences in free
energy
Table 17.1 presents a range of acids and their pKa
values](https://image.slidesharecdn.com/chapter171-230505164754-68d9a8ce/85/chapter17-1-ppt-10-320.jpg)

































![44
17.10 Reactions of Phenols
The hydroxyl group is a strongly activating, making
phenols substrates for electrophilic halogenation,
nitration, sulfonation, and Friedel–Crafts reactions
Reaction of a phenol with strong oxidizing agents
yields a quinone
Fremy's salt [(KSO3)2NO] works under mild
conditions through a radical mechanism](https://image.slidesharecdn.com/chapter171-230505164754-68d9a8ce/85/chapter17-1-ppt-44-320.jpg)