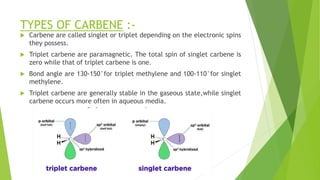
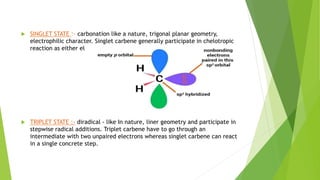
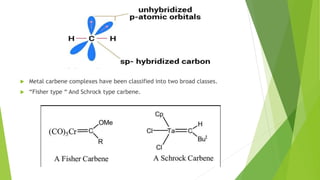
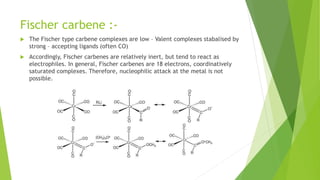
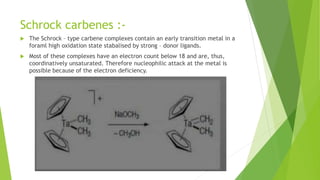
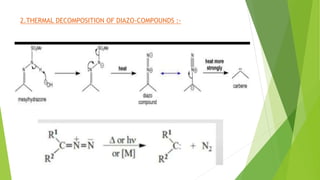
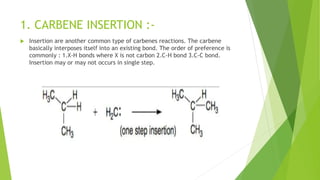
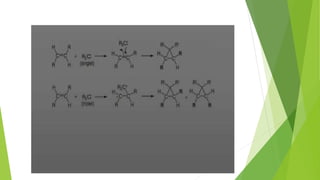
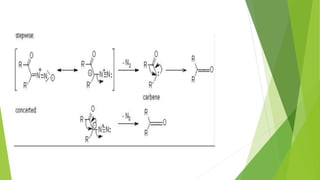
This document discusses carbenes, which are neutral carbon molecules with two unshared valence electrons. It describes the different types of carbenes, including singlet and triplet carbenes, and their electronic structures and bonding properties. Methods of forming carbenes are presented, such as alpha elimination reactions and decomposition of diazo compounds. The major reactions of carbenes are also summarized, including insertion, addition, and rearrangement reactions. Carbene reactivity depends on whether they are in singlet or triplet states.