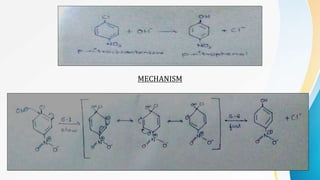
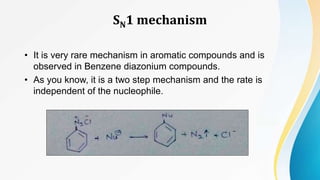
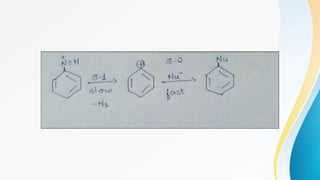
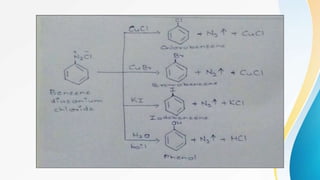
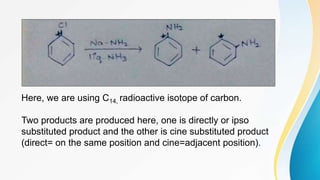
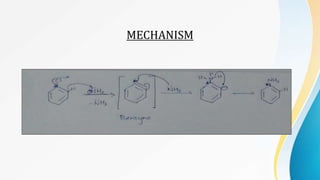
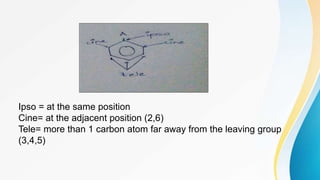
This document summarizes aromatic nucleophilic substitution reactions. It discusses the SNAr, SN1, and benzyne mechanisms. For SNAr, a strong withdrawing group is needed for reactivity. SN1 is rare for aromatics. In the benzyne mechanism, elimination of H forms benzyne which then adds the nucleophile, producing either ortho or para substituted products. Radiocarbon labeling is used to identify the products. In summary, the document outlines different aromatic nucleophilic substitution reaction mechanisms and factors affecting their reactivity.