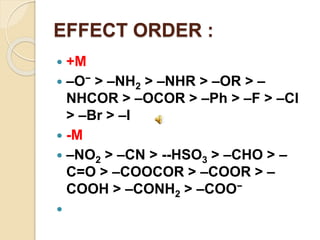
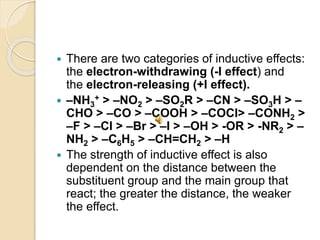
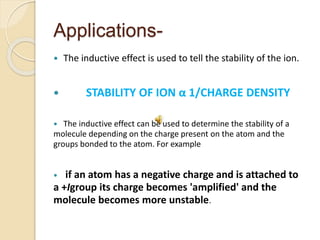
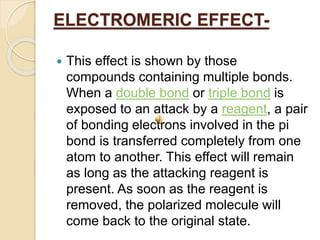
This document discusses different electronic effects in organic chemistry. It describes inductive effect as a permanent polarization of electron density between two unlike atoms in a bond. Electron-withdrawing and electron-releasing groups are discussed. Mesomeric effect allows for resonance stabilization through delocalization of charge in conjugated systems. Electromeric effect involves the temporary shift of a pi bond electron pair to one atom upon reaction with an electrophile. The order of effects from strongest to weakest is given as mesomeric, electromeric, and inductive. Applications to stability of ions and carbocations are outlined.







![MESOMERIC EFFECT-
The concepts of mesomeric effect,
mesomerism and mesomer were introduced
by Ingold in 1938 as an alternative to
Pauling's synonymous concept of
resonance.[1] "Mesomerism" in this context is
often encountered in German and French
literature, but in English literature the term
"resonance" dominates.
Mesomeric effect can be transmitted along
any number of carbon atoms in a conjugated
system. This accounts for the resonance
stabilization of the molecule due to
delocalization of charge.](https://image.slidesharecdn.com/electroniceffects-inductivemesomericelectromeric-201027151127/85/Electronic-effects-inductive-mesomeric-electromeric-11-320.jpg)