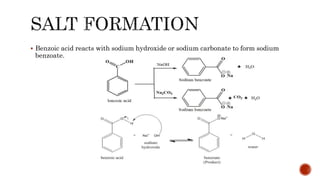
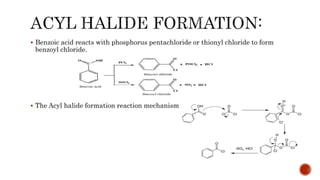
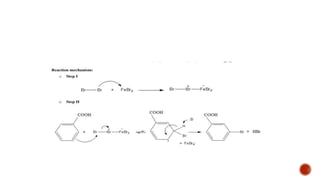
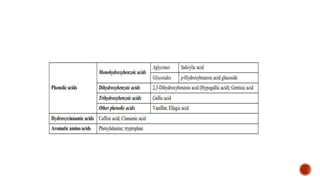
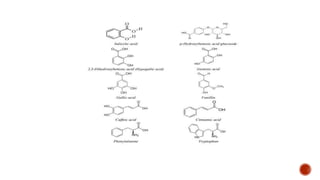
Benzoic acid and its derivatives have several uses:
(i) Benzoic acid is used as a food preservative and antiseptic. It can be converted to benzoyl chloride or sodium benzoate.
(ii) Salicylic acid is used as a food preservative, bactericide, and to treat warts, acne, and other skin conditions.
(iii) Gallic acid has antioxidant and anti-inflammatory properties and may help protect against cancer, heart disease, and neurological disorders.











![ Preferred IUPAC name: Benzoic acid
Systematic IUPAC name: Benzenecarboxylic acid
Other names : Carboxybenzene, Dracylic acid, Phenylmethanoic acid
Chemical formula : C7H6O2
Molar mass: 122.123 g·mol−1
Appearance: Colorless crystalline solid
Density :1.2659 g/cm3 (15 °C)
Melting point :122 °C
Solubility in water: Insoluble [1.7 g/L (0 °C); 2.7 g/L (18 °C); 3.44 g/L (25 °C); 5.51
g/L (40 °C); 21.45 g/L (75 °C); 56.31 g/L (100 °C)]
Solubility: soluble in acetone, benzene, CCl4, CHCl3, alcohol, ethyl ether, hexane,
phenyls, liquid ammonia, acetate](https://image.slidesharecdn.com/aromaticacidsvrm-230926045924-eb3268c2/85/AROMATIC-ACIDS-pptx-16-320.jpg)