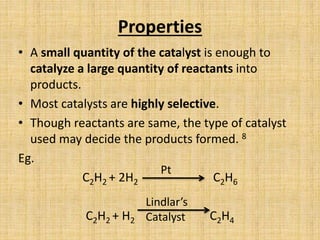
This document discusses the topic of catalysis through definitions, properties, and examples. It defines catalysis as a process where a substance called a catalyst speeds up a chemical reaction by lowering the activation energy without being consumed. Common catalysts include transition metals which provide vacant orbitals for substrate bonding. The document provides examples of catalysis in industrial chemical production and biological systems like photosynthesis. It also explains how catalysts work by providing an alternative reaction pathway requiring less energy.