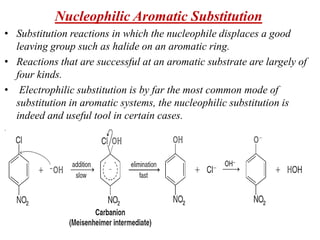
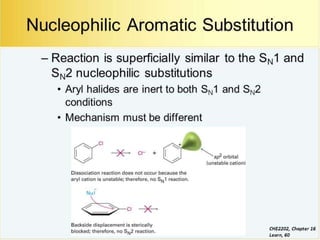
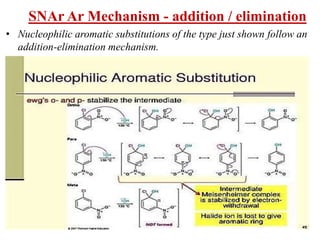
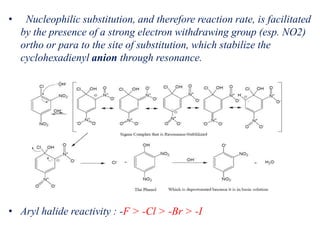
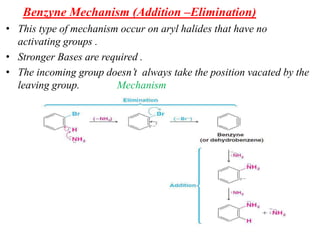
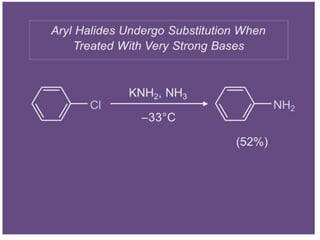
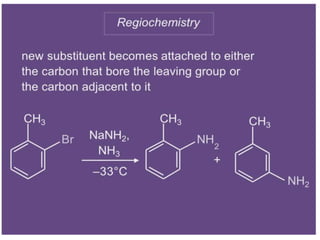
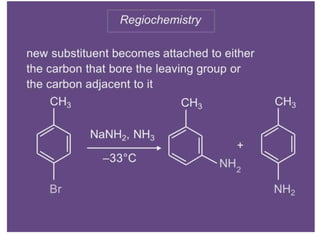
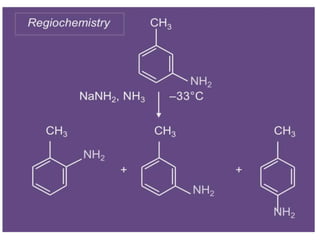
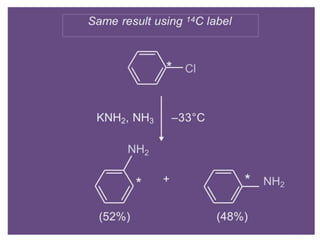
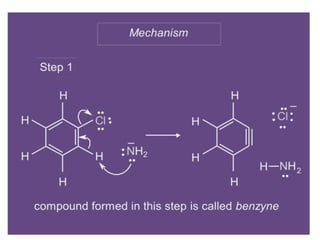
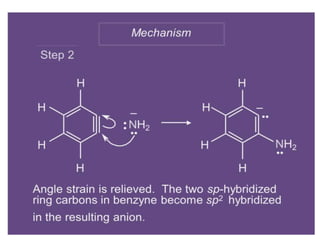
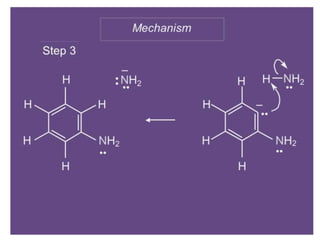
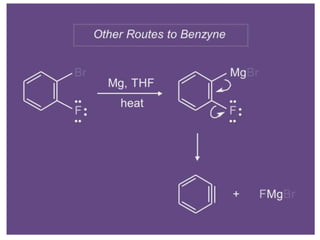
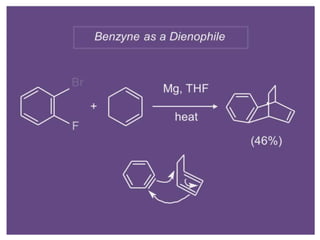
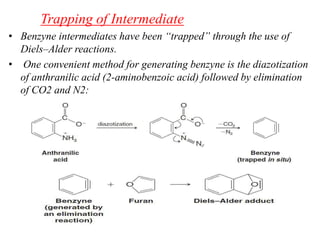
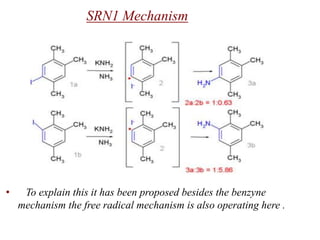
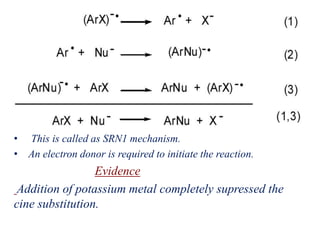
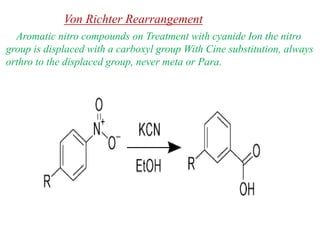
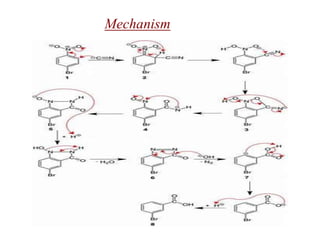
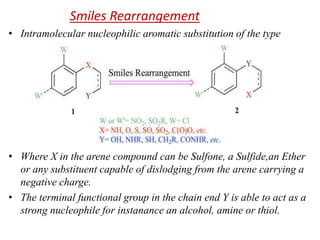
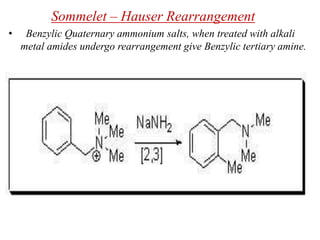
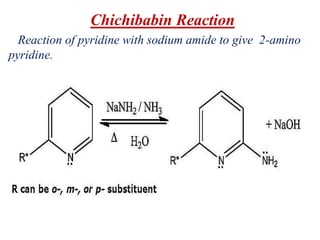
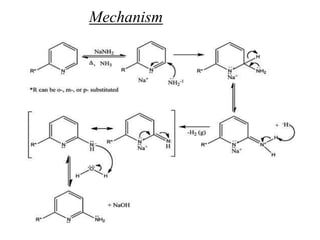
Nucleophilic aromatic substitution is a reaction where a nucleophile displaces a good leaving group such as a halide on an aromatic ring. The document discusses several mechanisms for nucleophilic aromatic substitution including SNAr, SN1, benzyne, SRN1, and examples like the Von Richter and Smiles rearrangements. The rate is facilitated by electron-withdrawing groups on the aromatic ring that stabilize the cyclohexadienyl anion intermediate.