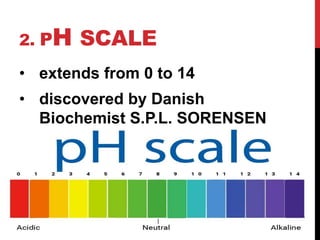
Acids produce H+ ions in water and taste sour, while bases produce OH- ions in water and taste bitter. Acids react with metals to produce hydrogen gas and with bases to form salts and water. The pH scale ranges from 0-14 and is used to measure whether a substance is acidic (below 7) or basic (above 7). Common indicators like litmus paper and the pH scale can be used to identify substances as acidic or basic. Maintaining the proper pH is important for processes like food preservation, plant growth, and human bodily functions.