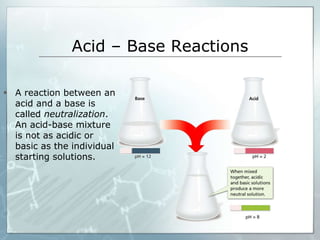
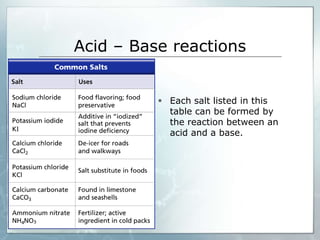
This document defines acids and bases, describing their properties and common uses. Acids have a pH below 7 and taste sour, while bases have a pH above 7 and feel soapy. The document explains that acids and bases react through neutralization, forming less acidic or basic salt mixtures. Common acids include acetic, citric, and sulfuric acids, which are found in vinegar, fruits, and industrial chemicals, while bases are found in soaps, cleaners, and the human body.