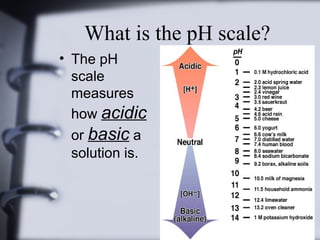
This document discusses acids and bases. It defines acids as substances that produce hydrogen ions (H+) in aqueous solutions, with a pH range of 0-7. Bases, also called alkalines, produce hydroxide ions (OH-) in aqueous solutions with a pH range of 7-14. A pH of 7 indicates a neutral substance. The goals are to determine the differences between acids and bases, discuss their importance, and perform an experiment testing the pH of various substances.


![The pH scale
• The pH scale is the concentration
of hydrogen ions in a given
substance.
[ ]
pH = − log H
+](https://image.slidesharecdn.com/acidsbases-140309061645-phpapp01/85/Acids-bases-4-320.jpg)

![[OH ]
−
Definitions of Acids and Bases
• An acid is a substance that breaks
+
into [ H ] ions in an aqueous
solution.
• A Base (alkaline) is a substance
that breaks into [OH − ] ions in an
aqueous solution.
• Note: aqueous solution is any
solution where H 2 O is the
solvent.](https://image.slidesharecdn.com/acidsbases-140309061645-phpapp01/85/Acids-bases-6-320.jpg)





