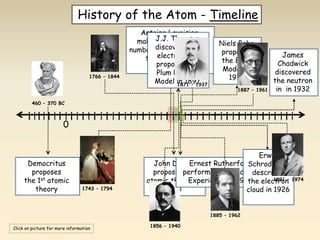
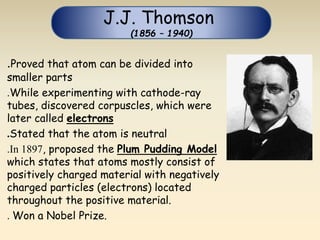
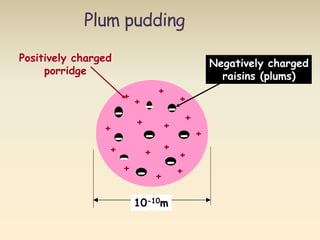
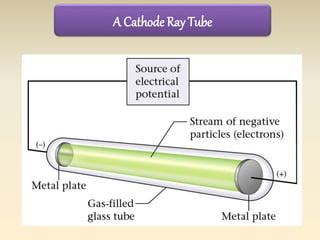
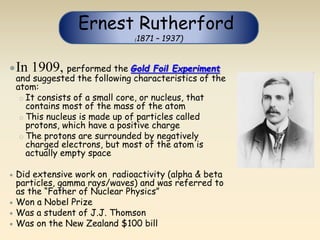
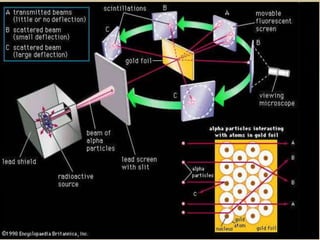
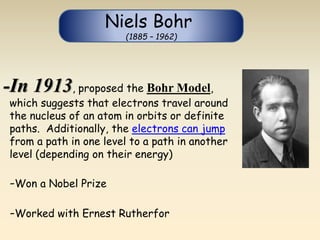
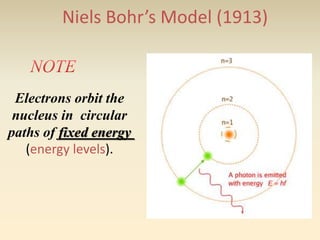
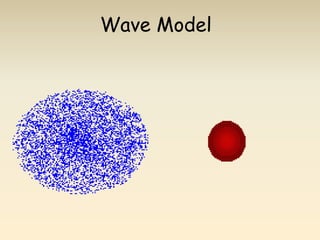
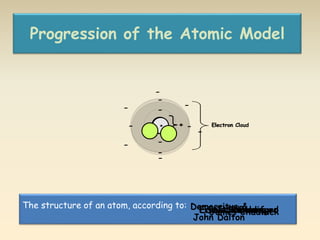
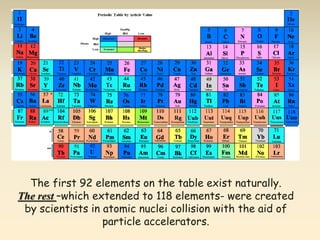
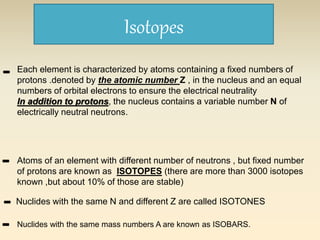
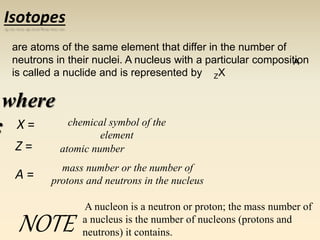
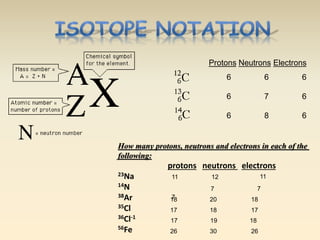
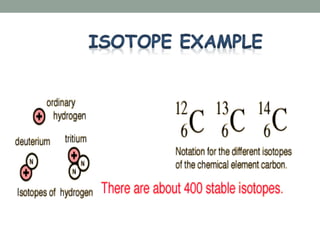
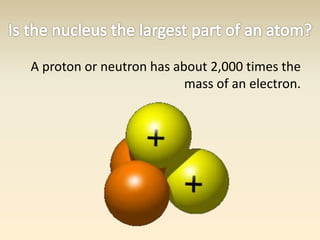
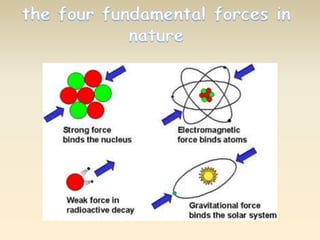
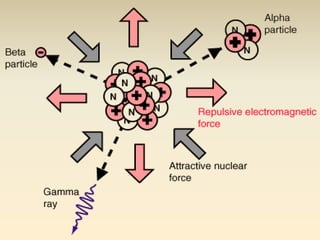
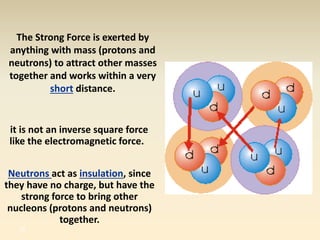
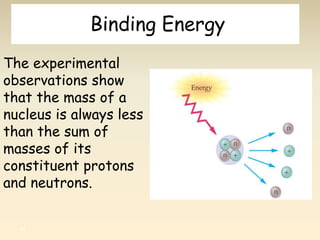
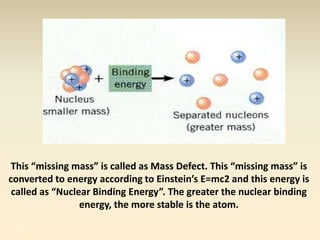
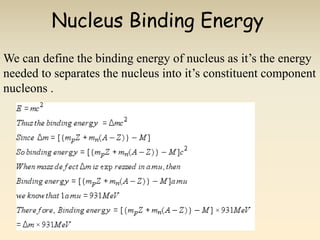
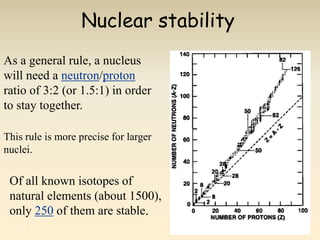
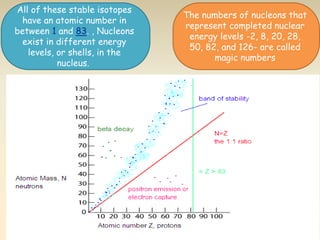
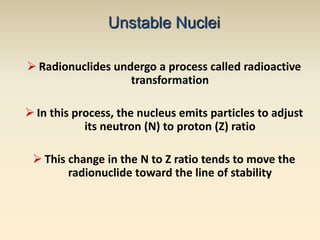
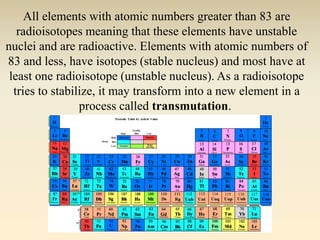
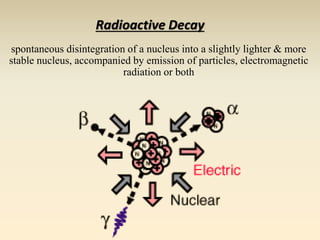
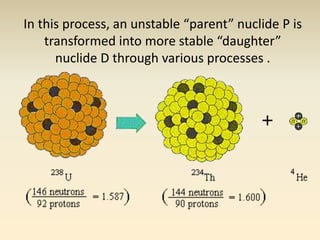
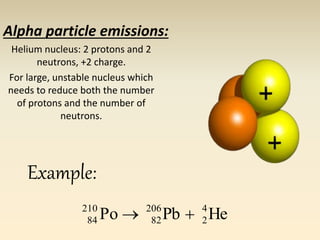
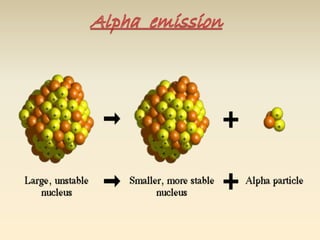
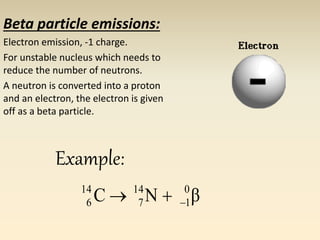
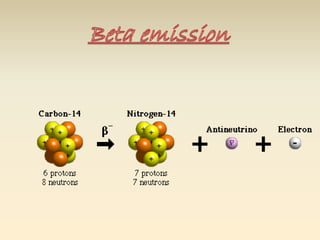
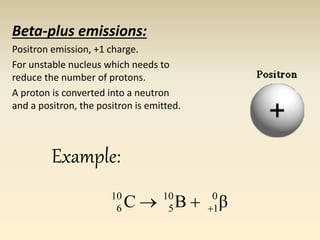
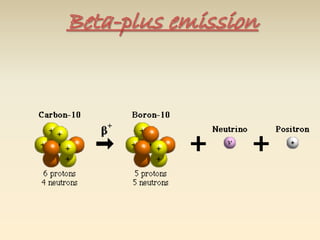
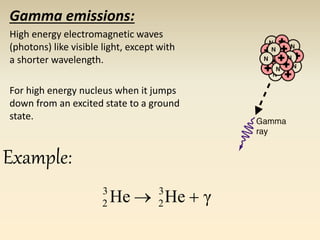
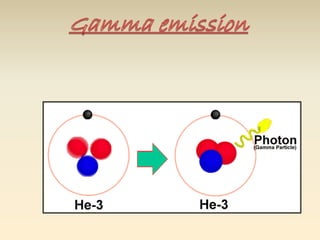
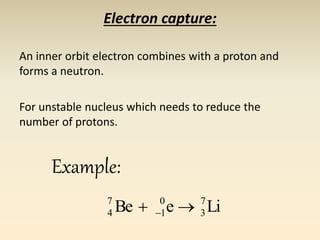
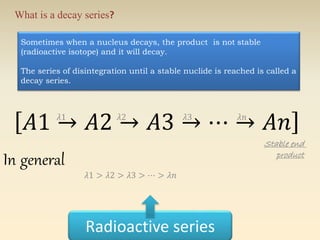
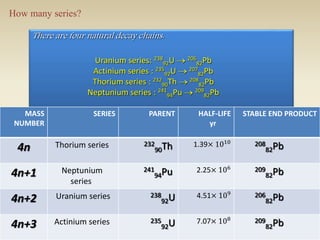
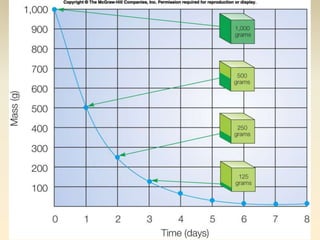
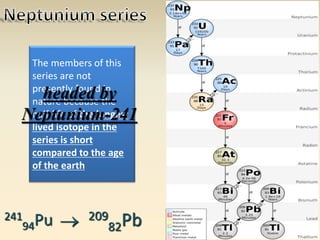
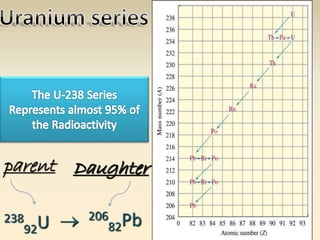
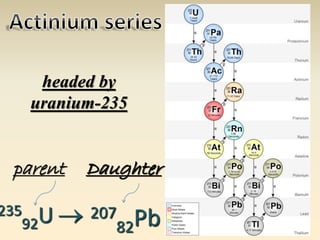
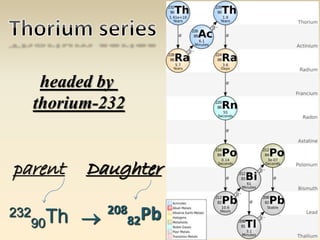
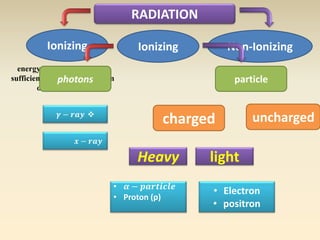
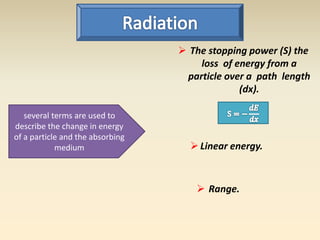
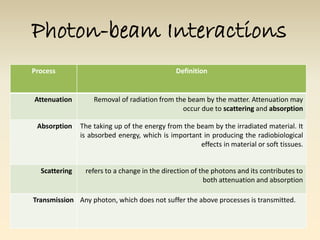
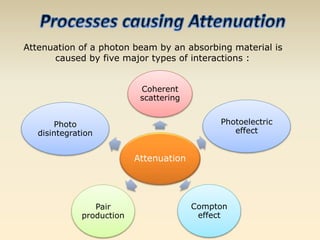
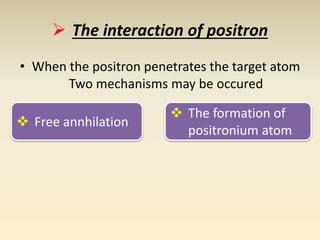
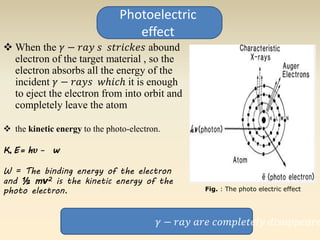
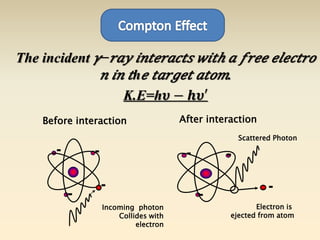
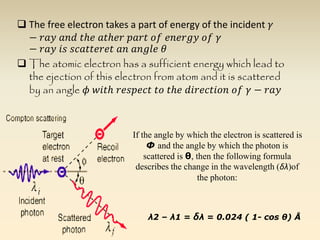
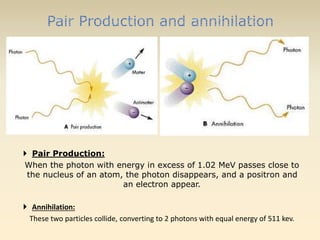
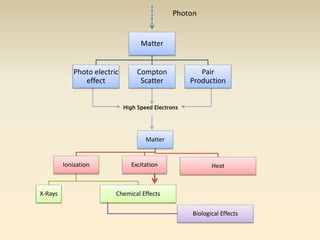
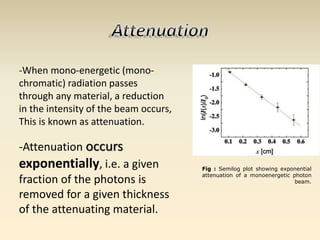
- The atomic model has evolved over time based on new evidence and experiments. Early thinkers proposed ideas including Democritus' atomic theory of small indivisible particles. John Dalton later proposed atoms of different elements have distinct properties. J.J. Thomson discovered the electron and proposed the plum pudding model. Rutherford's gold foil experiment showed the atom's small, dense nucleus. Niels Bohr incorporated electron orbits into his model. Later, Schrodinger and others developed the concept of electron clouds. Chadwick discovered the neutron in 1932, completing the standard atomic model.