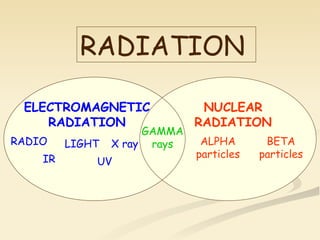
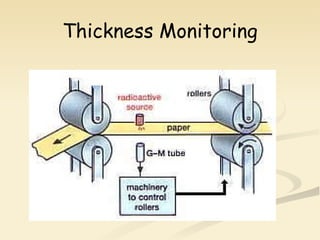
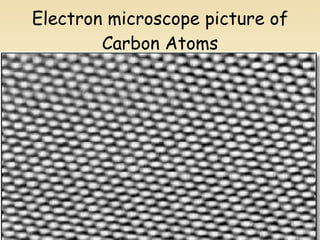
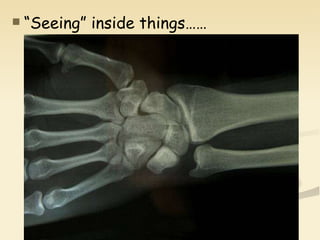
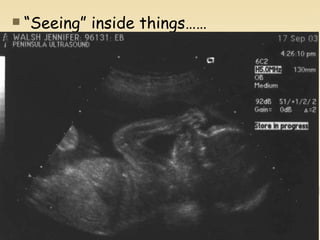
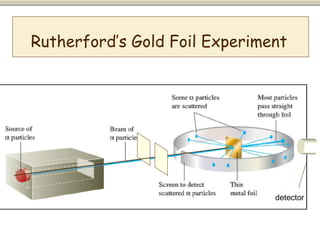
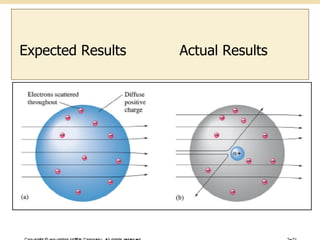
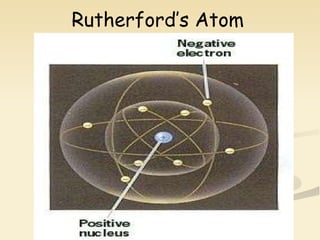
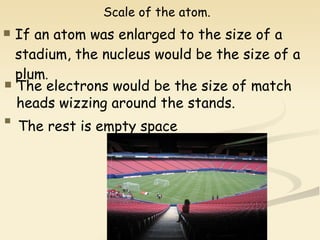
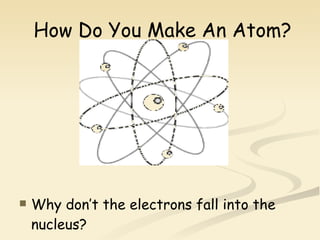
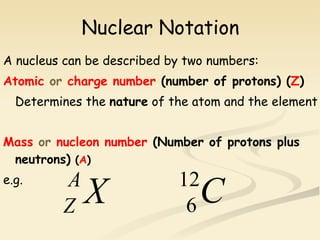
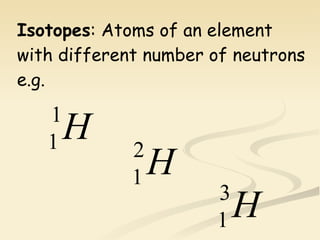
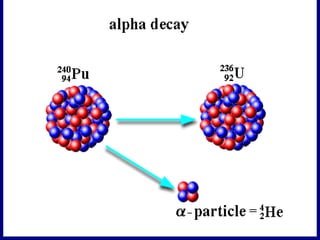
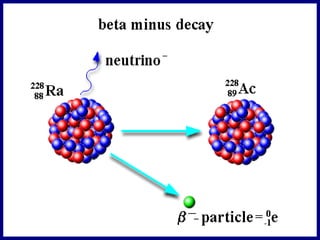
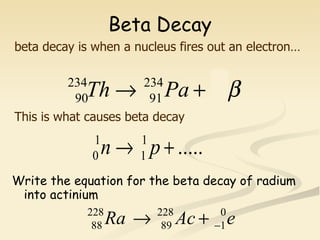
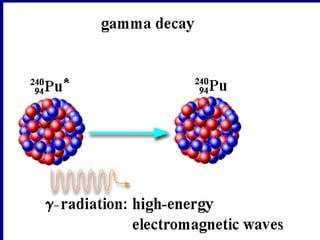
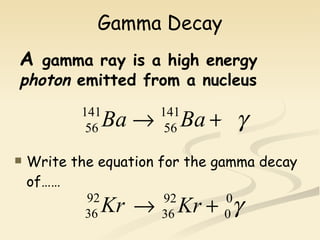
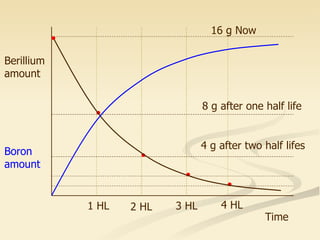
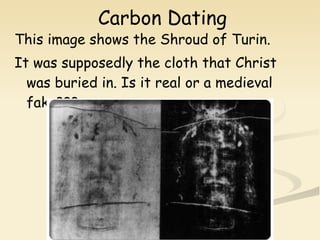
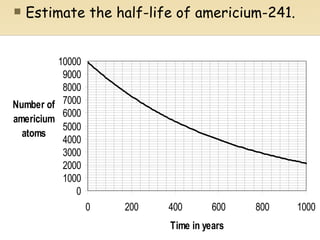
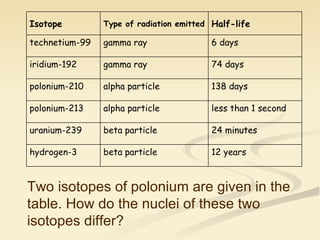
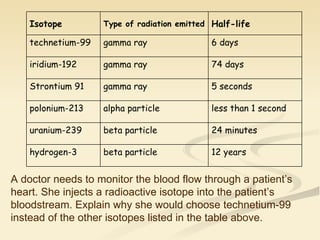
Nuclear physics involves understanding atoms through experiments like Rutherford's gold foil experiment which showed that atoms have a small, dense nucleus surrounded by empty space. Radiation like alpha, beta, and gamma rays is used in applications such as cancer treatment, electricity generation, and radiocarbon dating which relies on the radioactive decay of carbon-14 to determine the age of ancient materials.