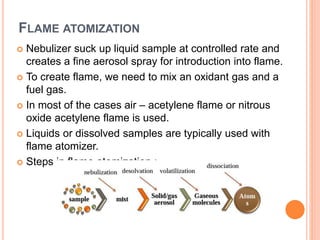
Atomic absorption spectroscopy is an analytical technique that measures the concentration of elements by detecting the amount of light absorbed by atoms in the gaseous state at specific wavelengths. It works by vaporizing and atomizing samples using a flame or graphite furnace, then measuring the absorption of light from a hollow cathode lamp at characteristic wavelengths. The instrument consists of a light source, atomizer, monochromator, detector, and readout system. Calibration curves of concentration versus absorption are used to determine unknown concentrations in samples. Potential interferences can affect the analysis and must be minimized. Atomic absorption spectroscopy has various applications in fields like metallurgy, pharmaceutical analysis, and biochemical analysis.