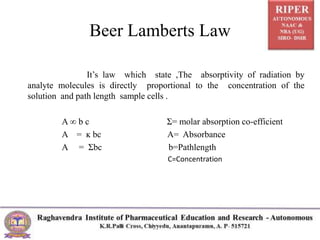
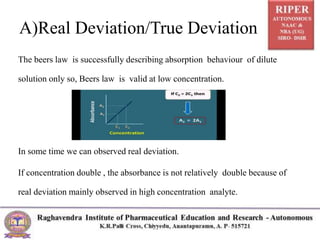
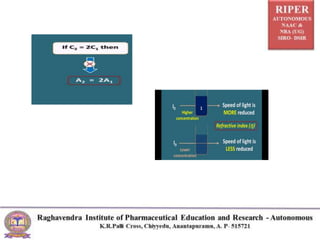
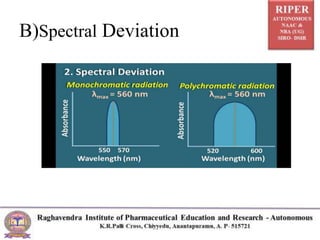
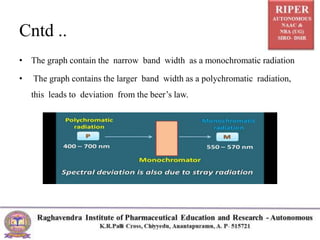
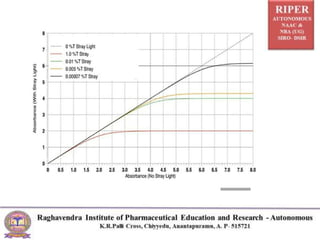
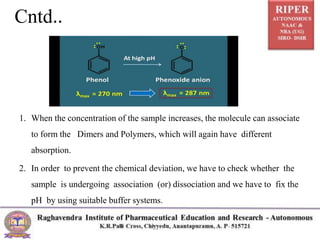
The document discusses Beer's Law and its deviations, which occur in different concentration ranges and under various conditions. It outlines three types of deviations: real, spectral, and chemical, detailing their causes and implications on absorption behavior. Additionally, it highlights applications of Beer's Law in drug concentration estimation and qualitative and quantitative analysis.