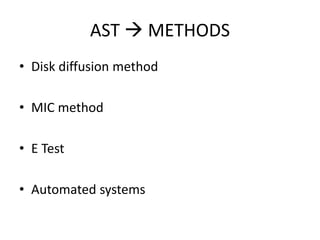
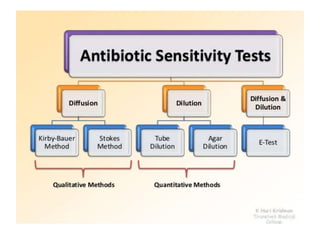
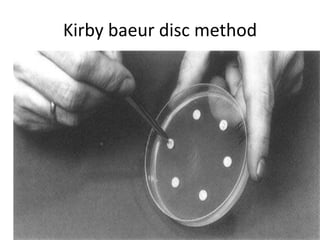
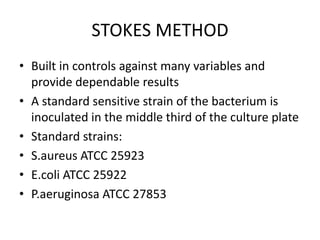
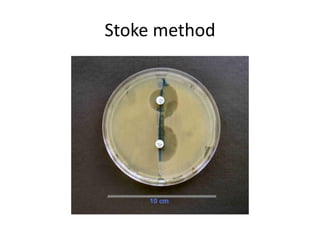
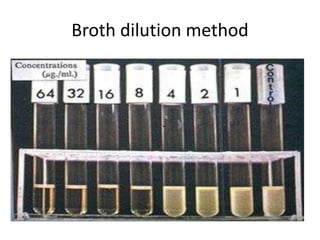
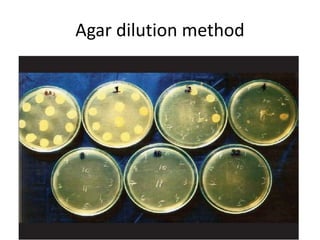
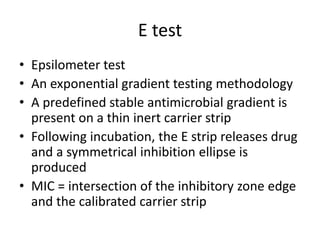
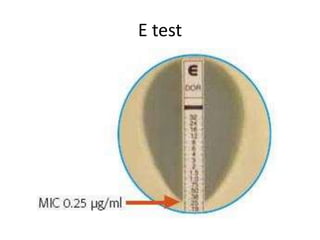
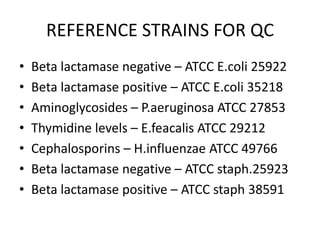
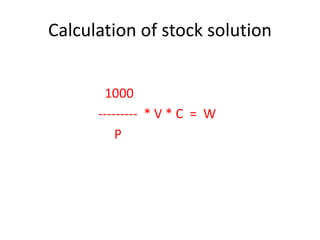Antimicrobial susceptibility testing (AST) determines the resistance of microorganisms to antimicrobial agents. There are several methods for AST including disk diffusion, dilution methods, and E tests. Guidelines for AST are provided by CLSI and EUCAST. Mueller Hinton agar is the recommended medium for testing and inoculum preparation follows the 0.5 McFarland standard. Quality control using standard reference strains is important to ensure accurate and reproducible AST results.