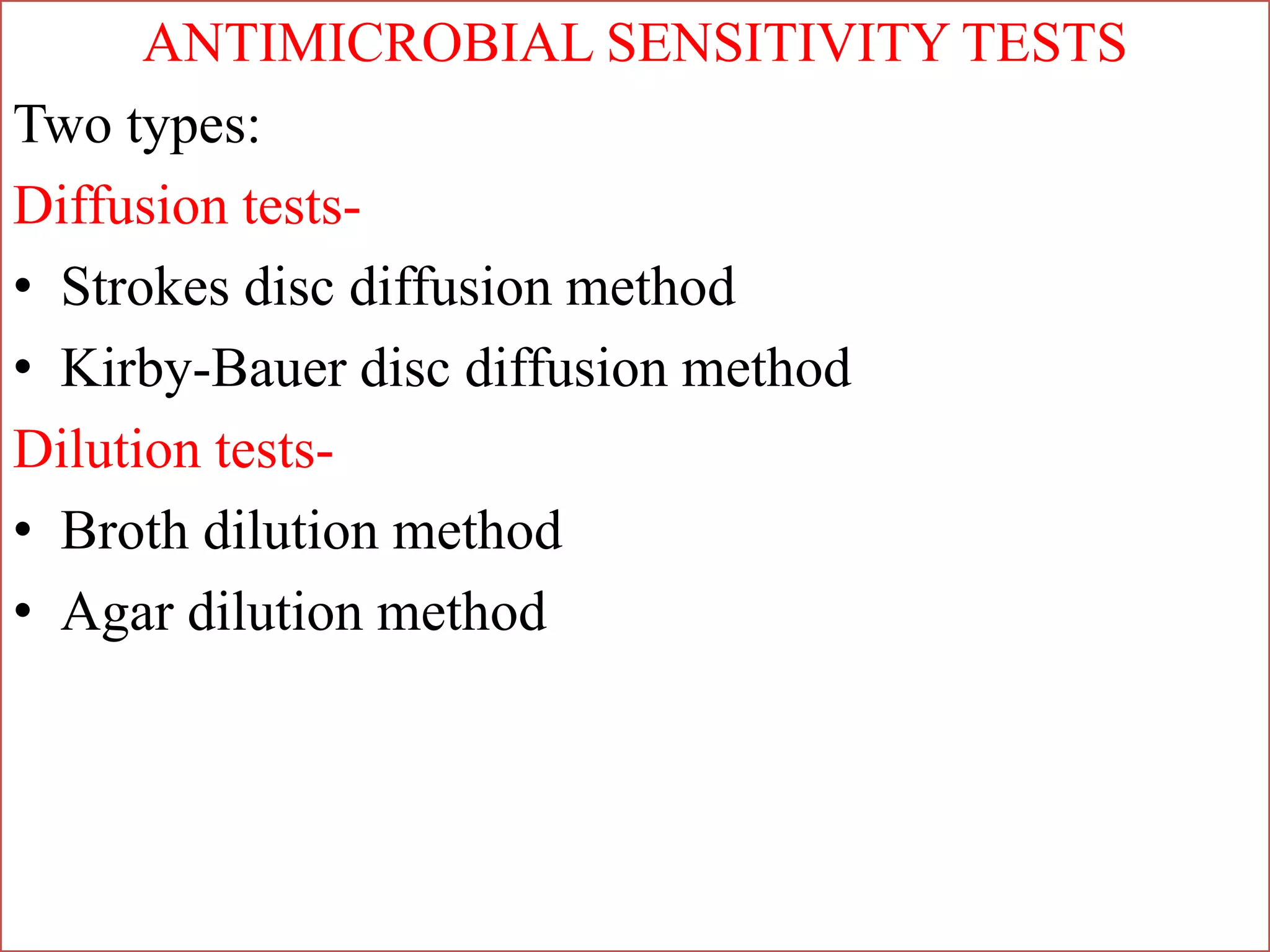
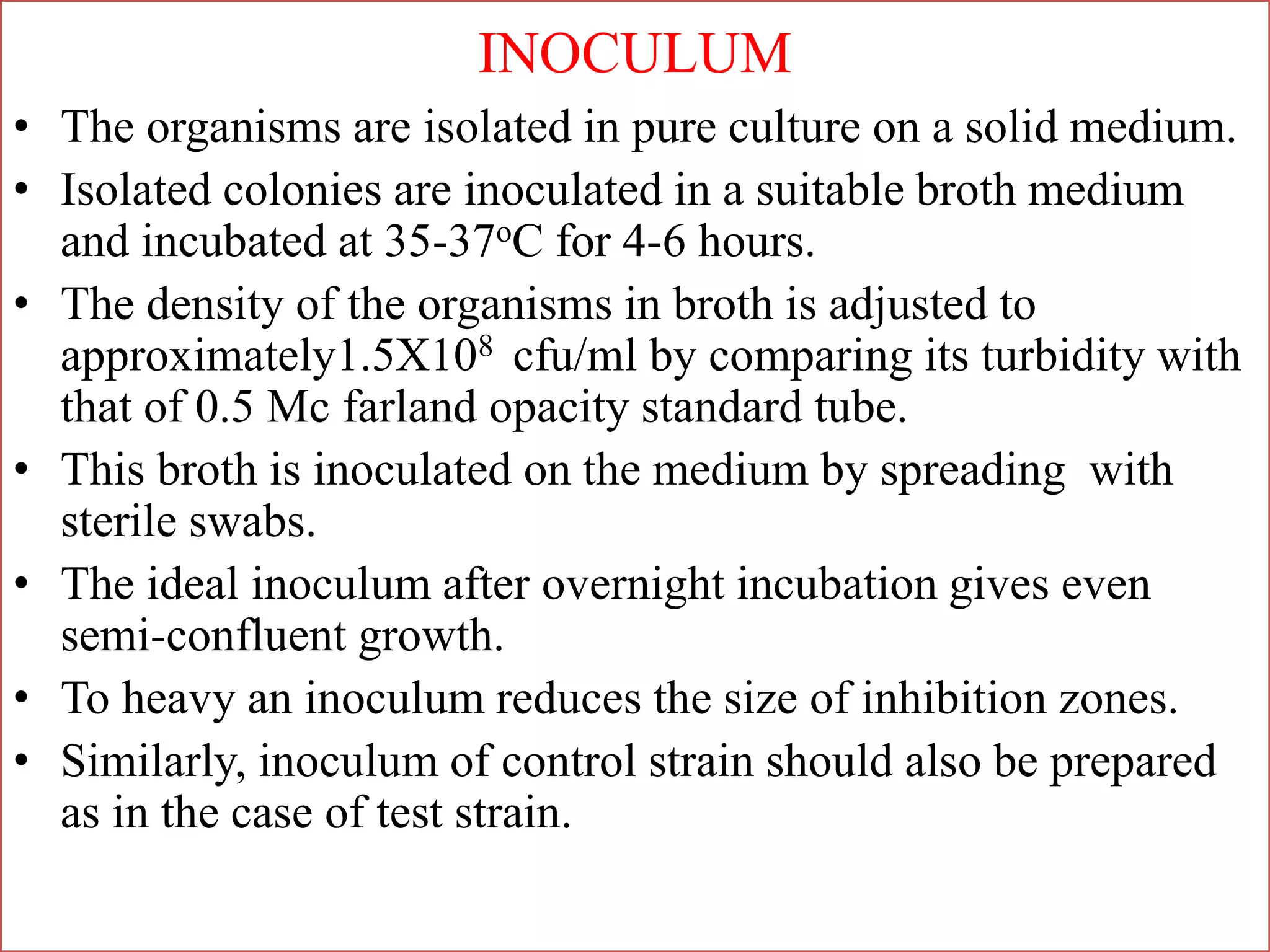
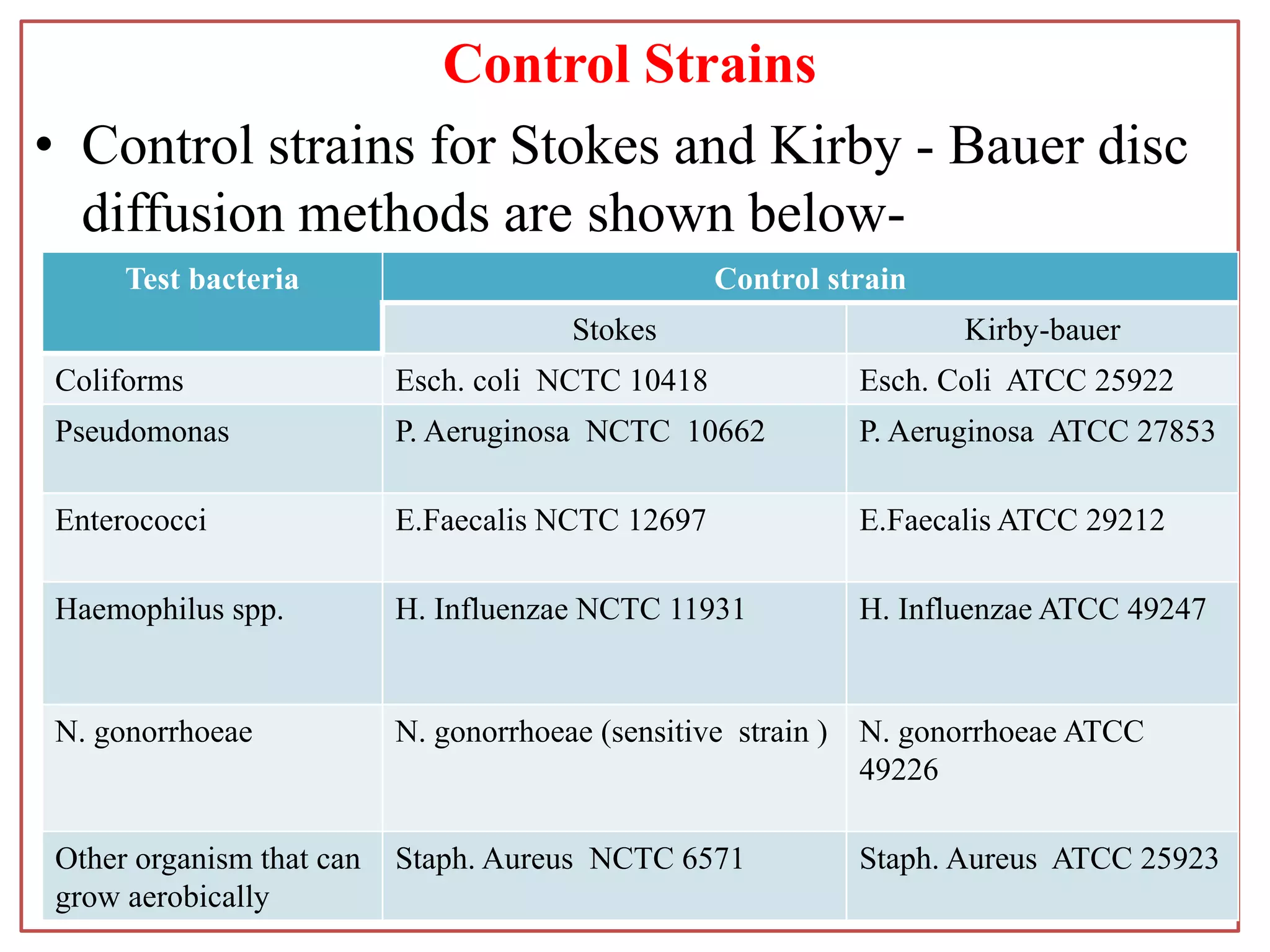
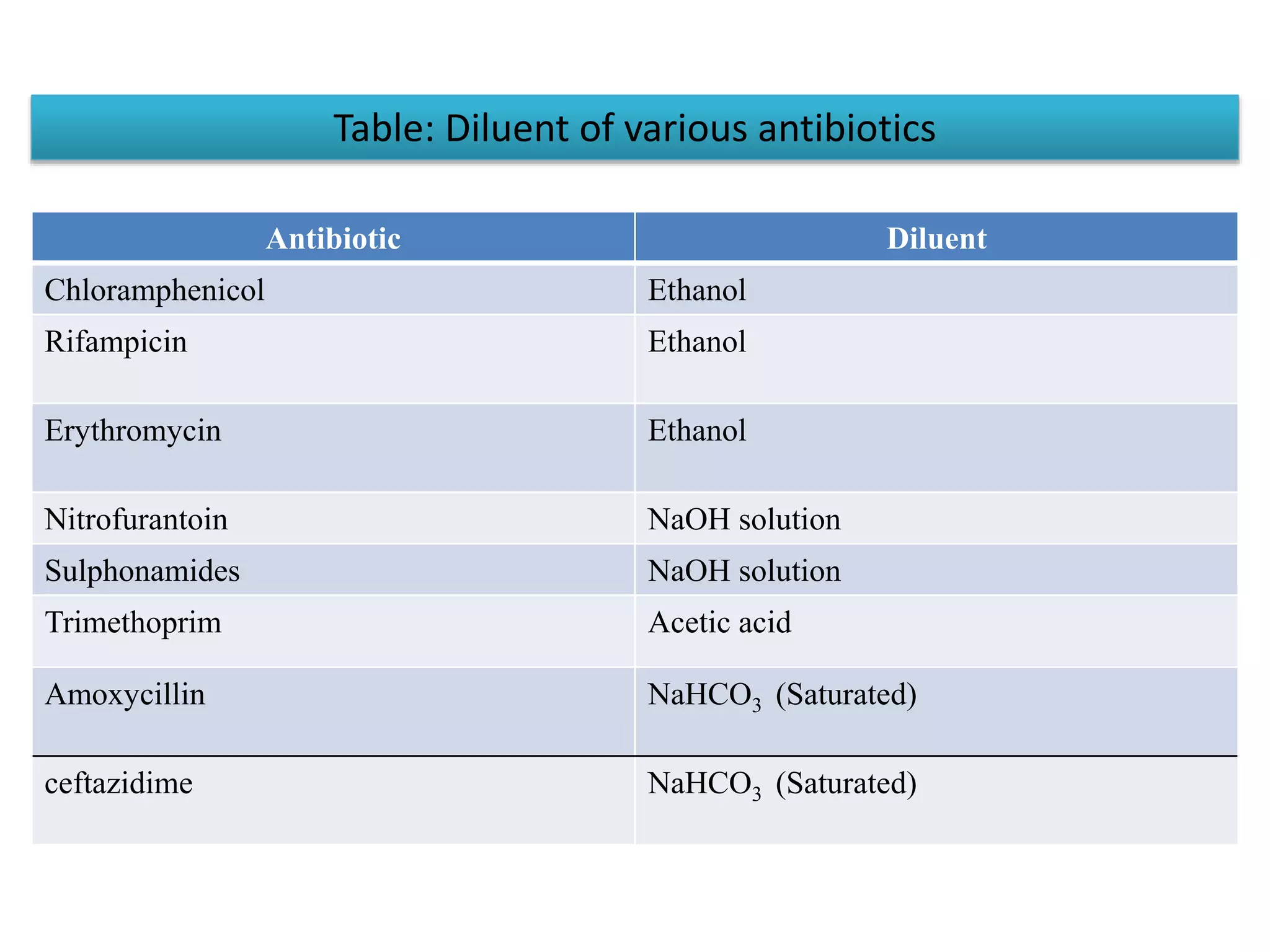
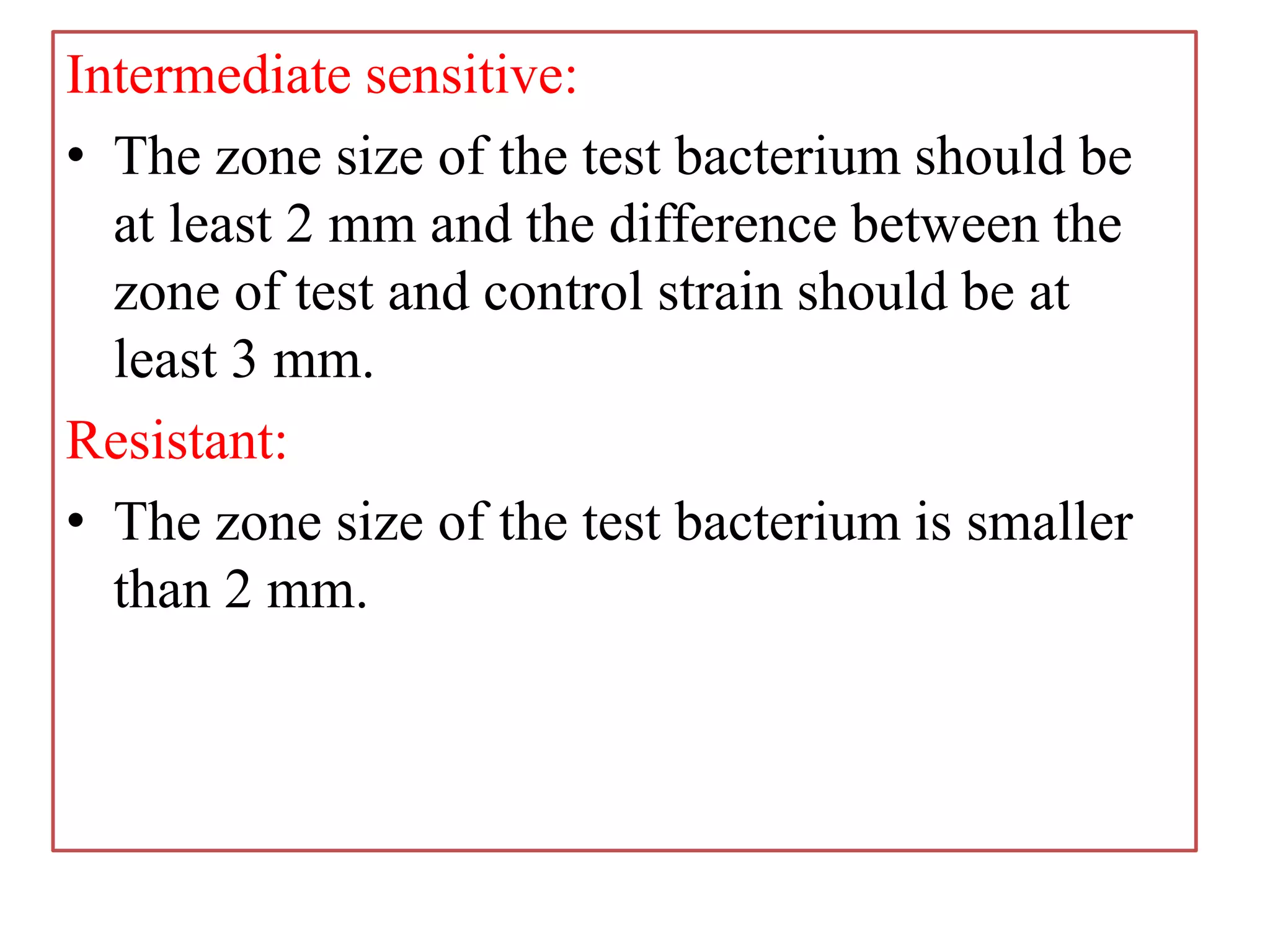
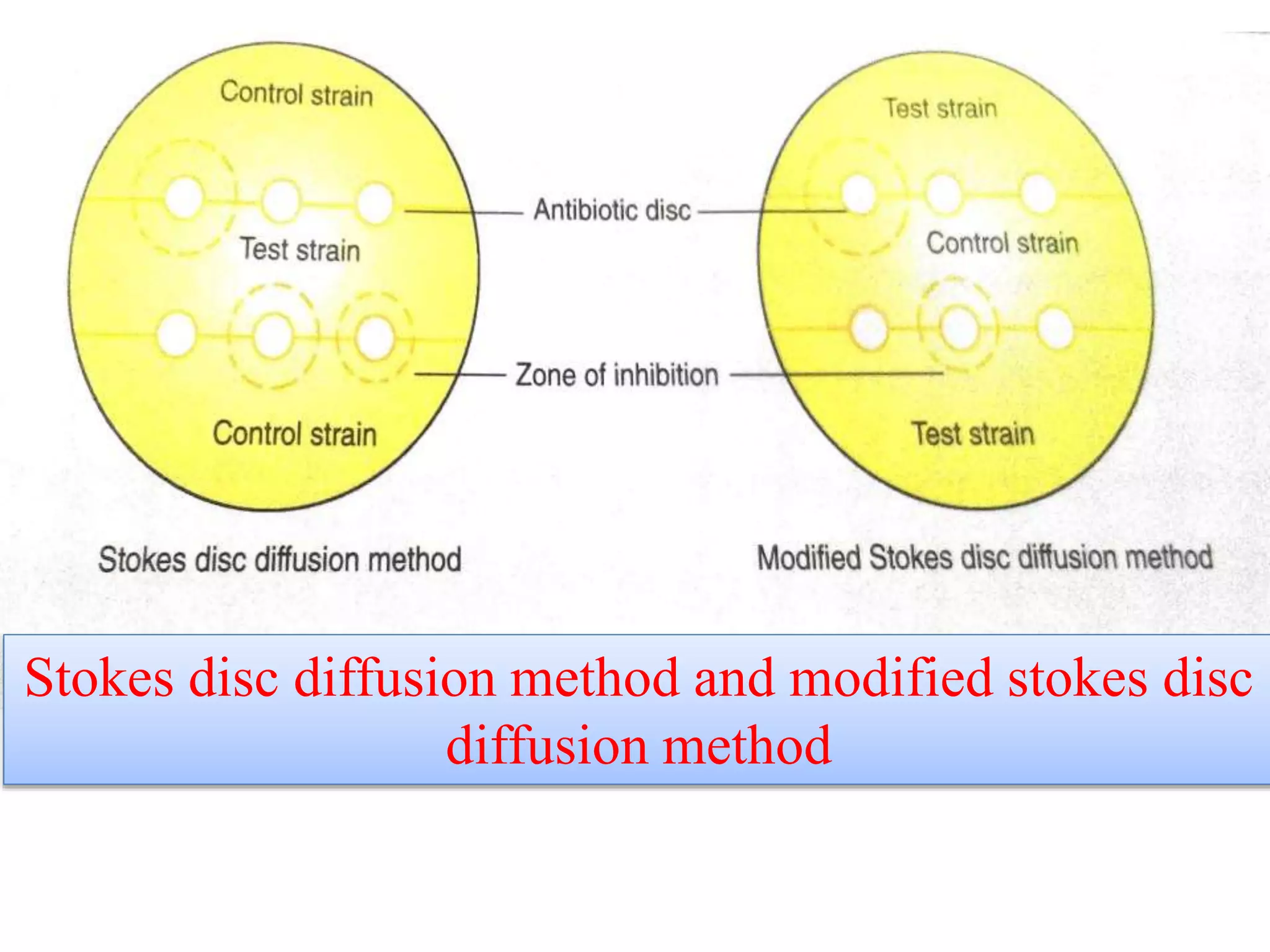
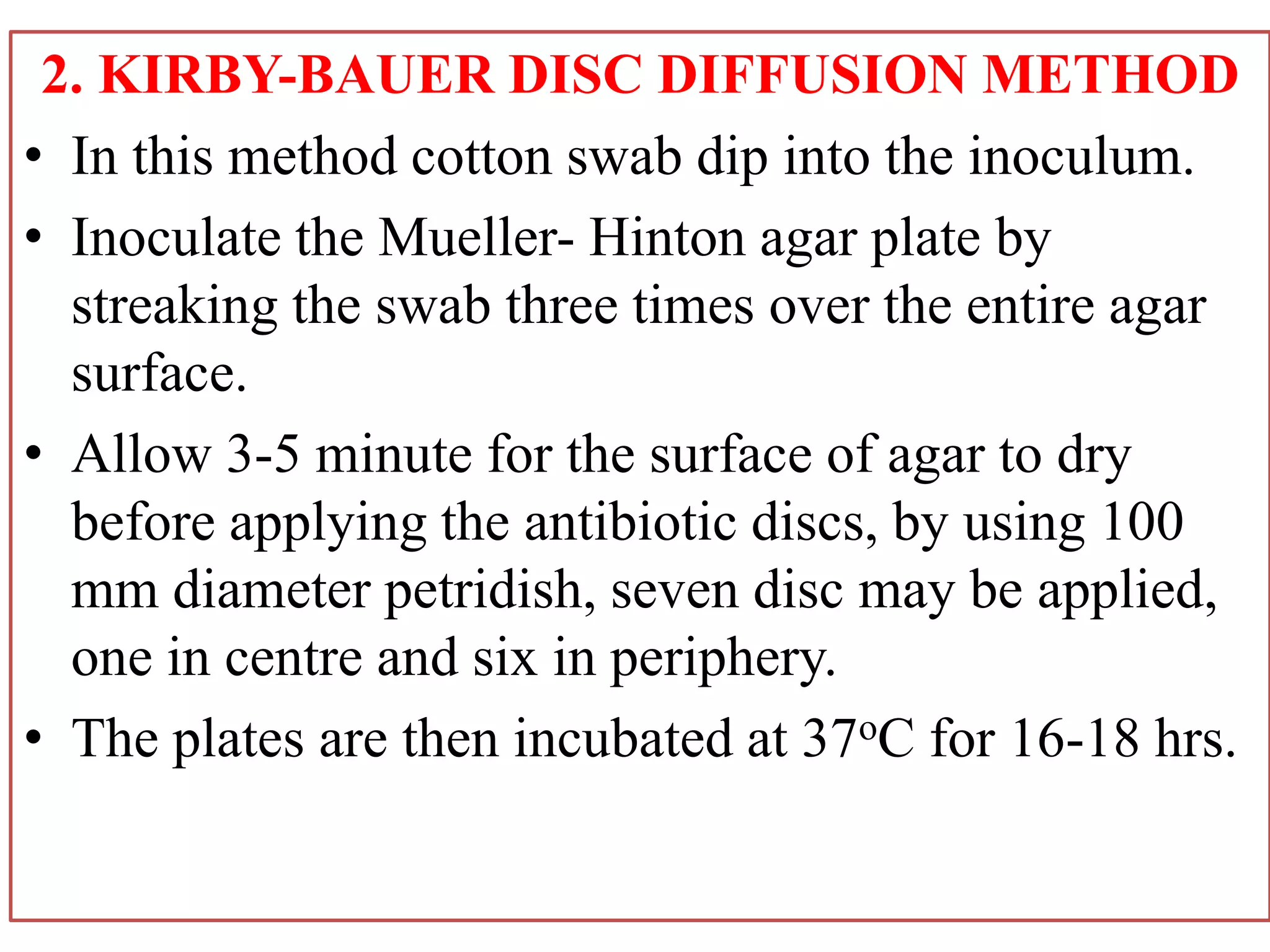
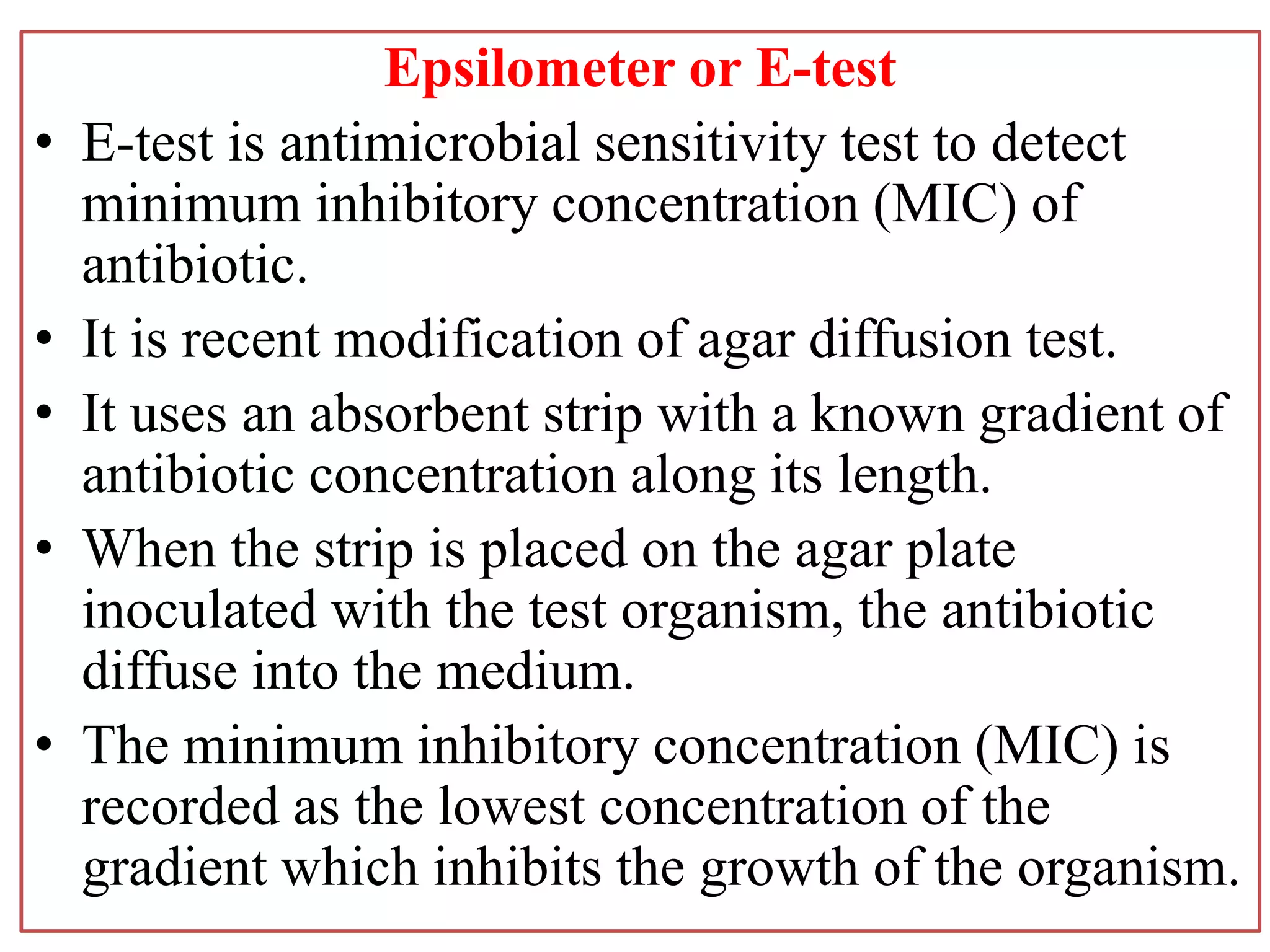
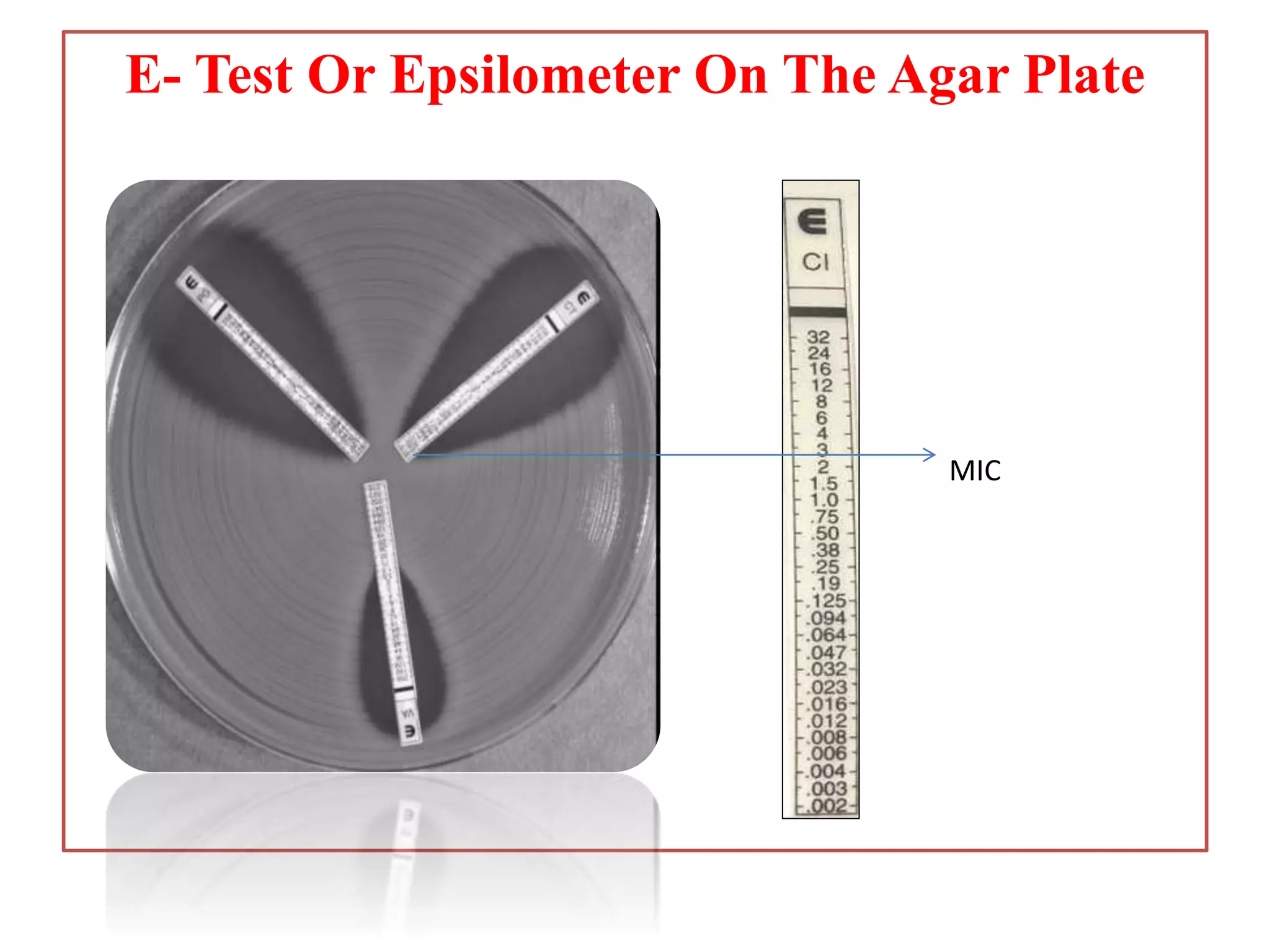
This document provides information on antibiotic sensitivity testing methods. It discusses the key terms related to antibiotic sensitivity like bacteriostatic, bactericidal, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC). It then describes the two main types of antibiotic sensitivity tests - diffusion tests like Kirby-Bauer disk diffusion method and dilution tests like broth dilution method. For diffusion tests, it explains how to prepare media, inoculum, antibiotic discs and controls and interpret the results. It also discusses Epsilometer or E-test method to detect MIC. For dilution tests, it details the broth dilution method to determine MIC and MBC.