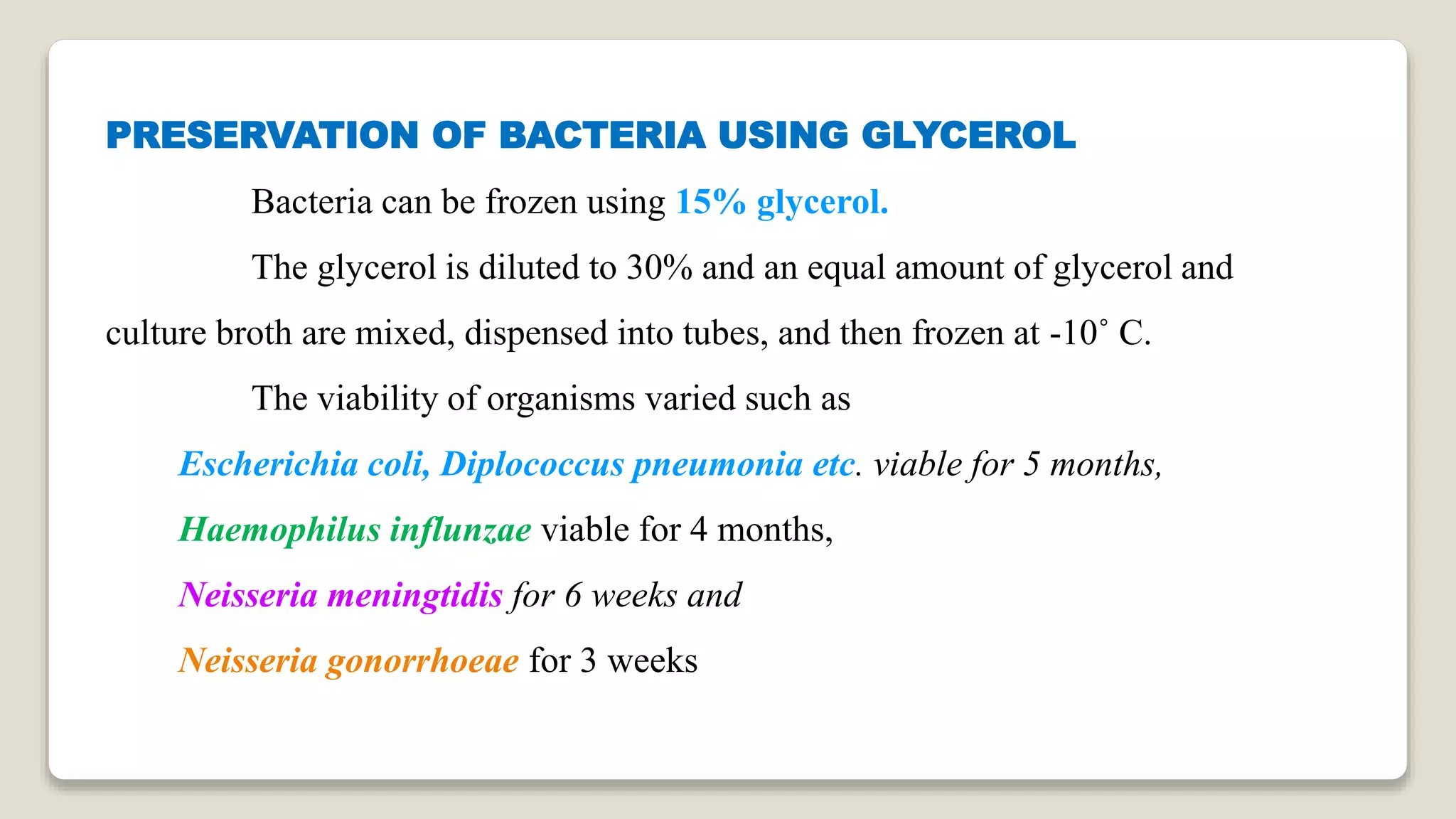
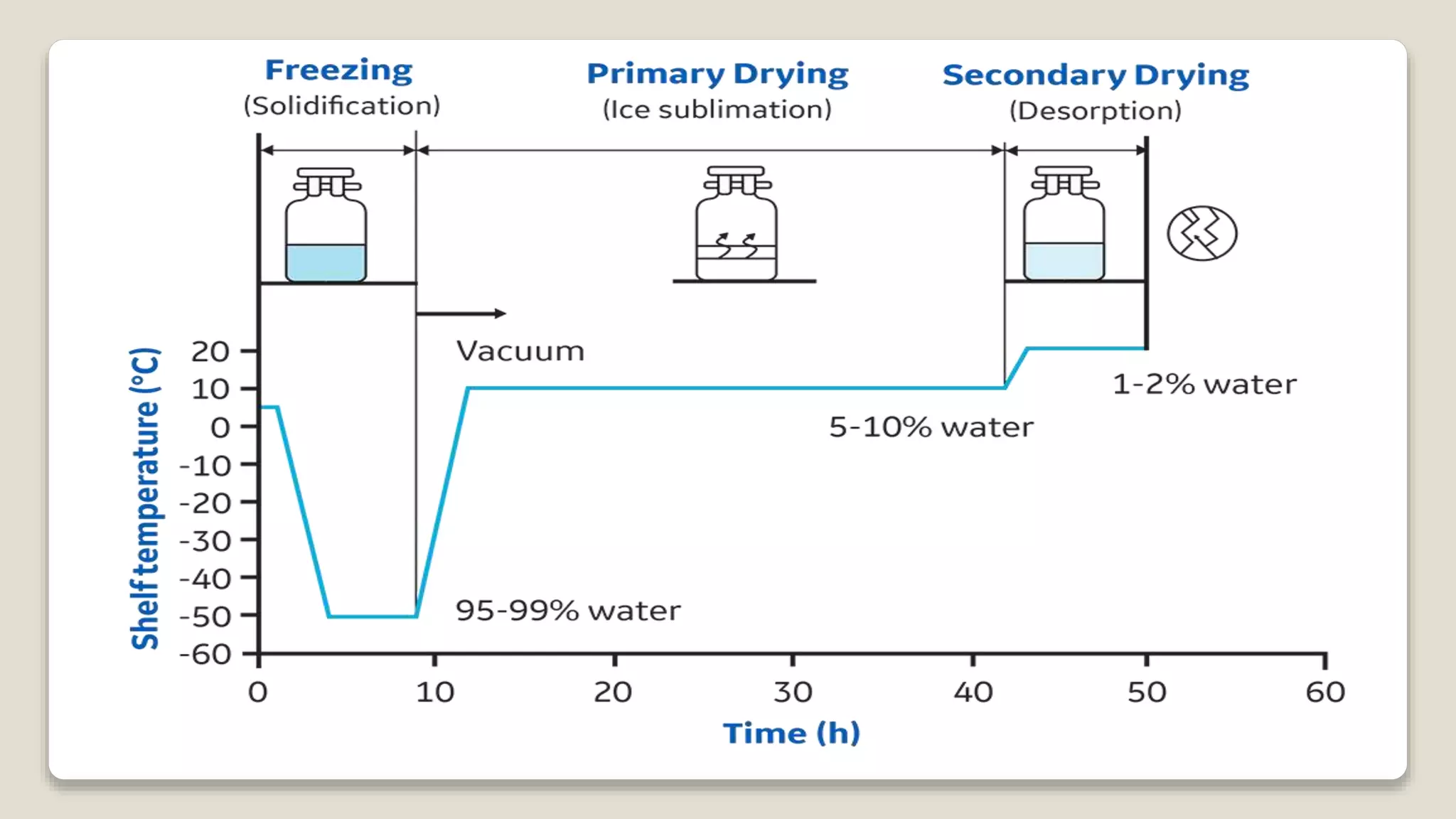
The document discusses various methods for preserving microorganisms. Short term methods include periodic transfer to fresh medium, storage in saline suspension, and refrigeration. Long term methods involve storage under mineral oil, lyophilization (freeze drying), cryopreservation in liquid nitrogen, and storage in sterile soil or silica gel. Lyophilization works by freezing and then reducing moisture content through sublimation and desorption. It allows storage at room temperature for many years but can damage some microbes. Cryopreservation in liquid nitrogen at -196°C also enables long term storage of over 10-30 years without genetic change.