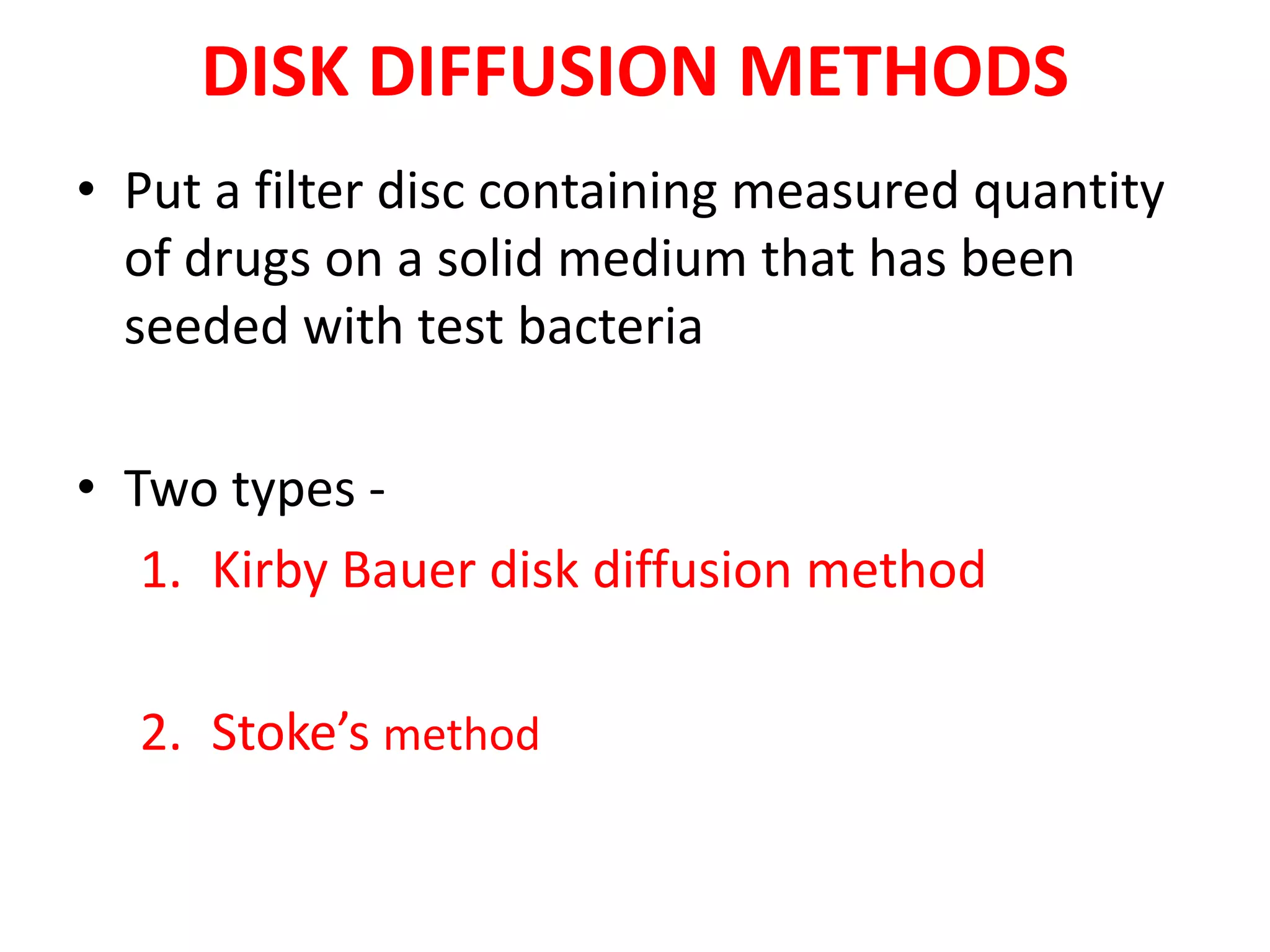
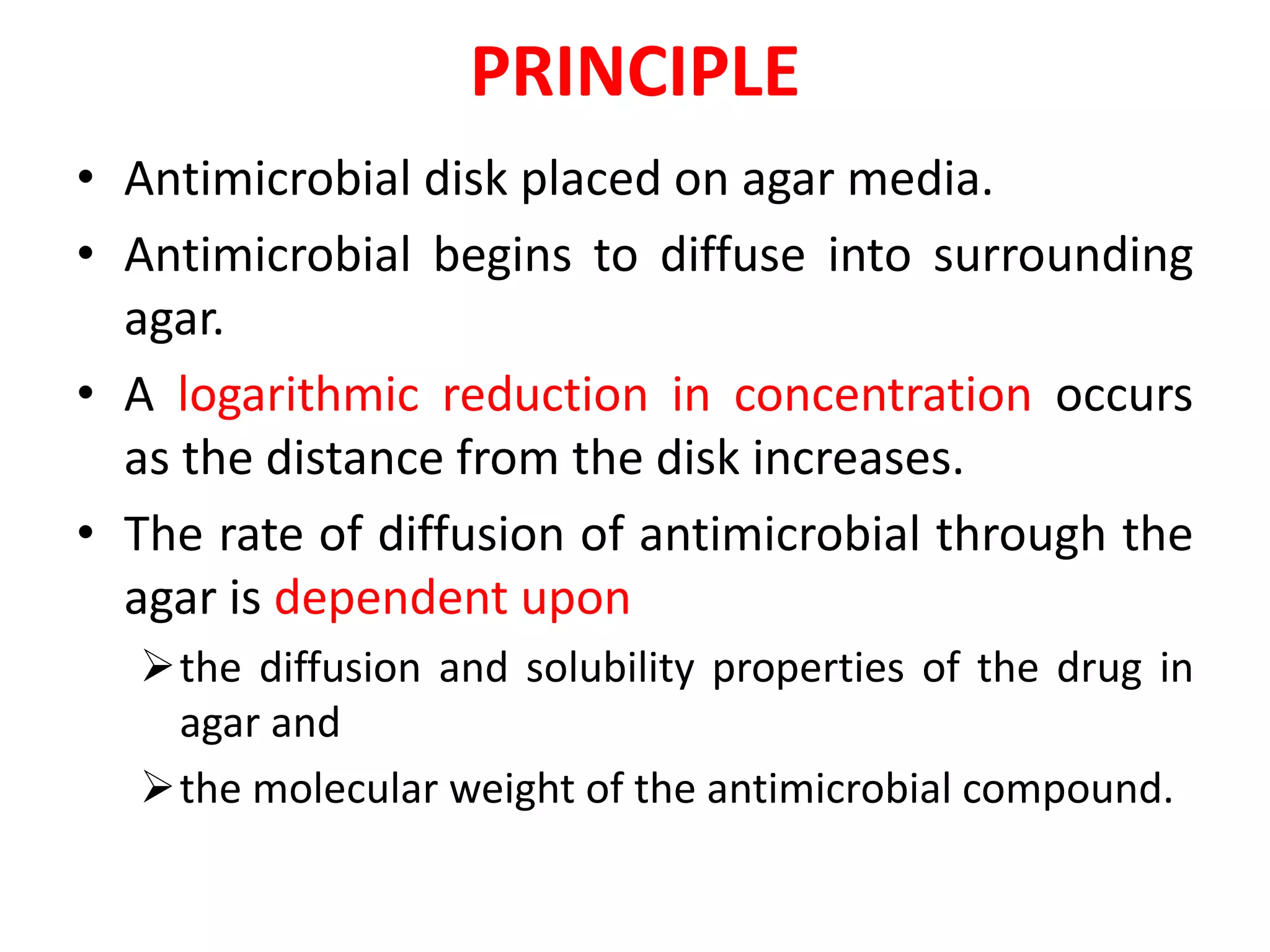
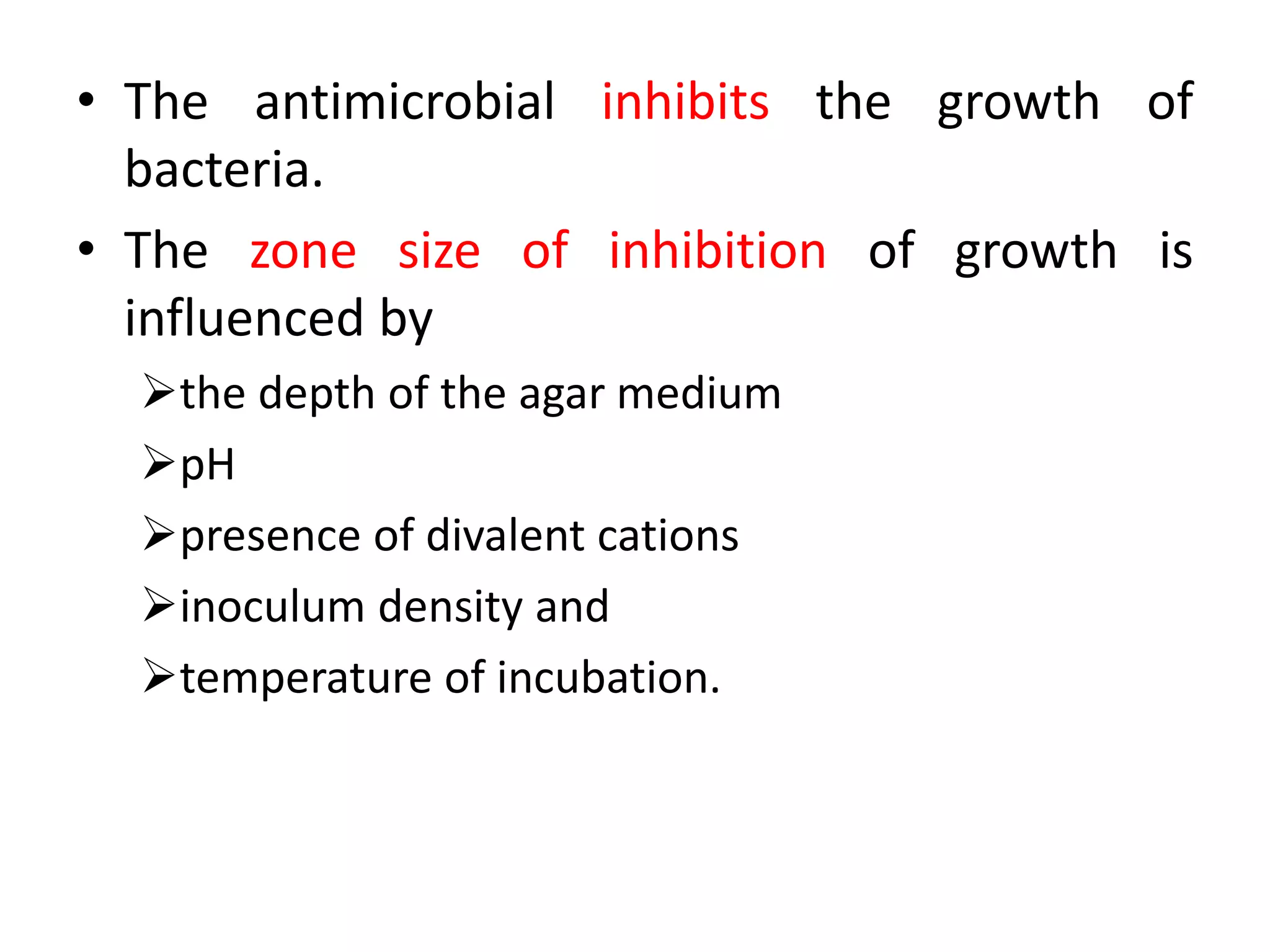
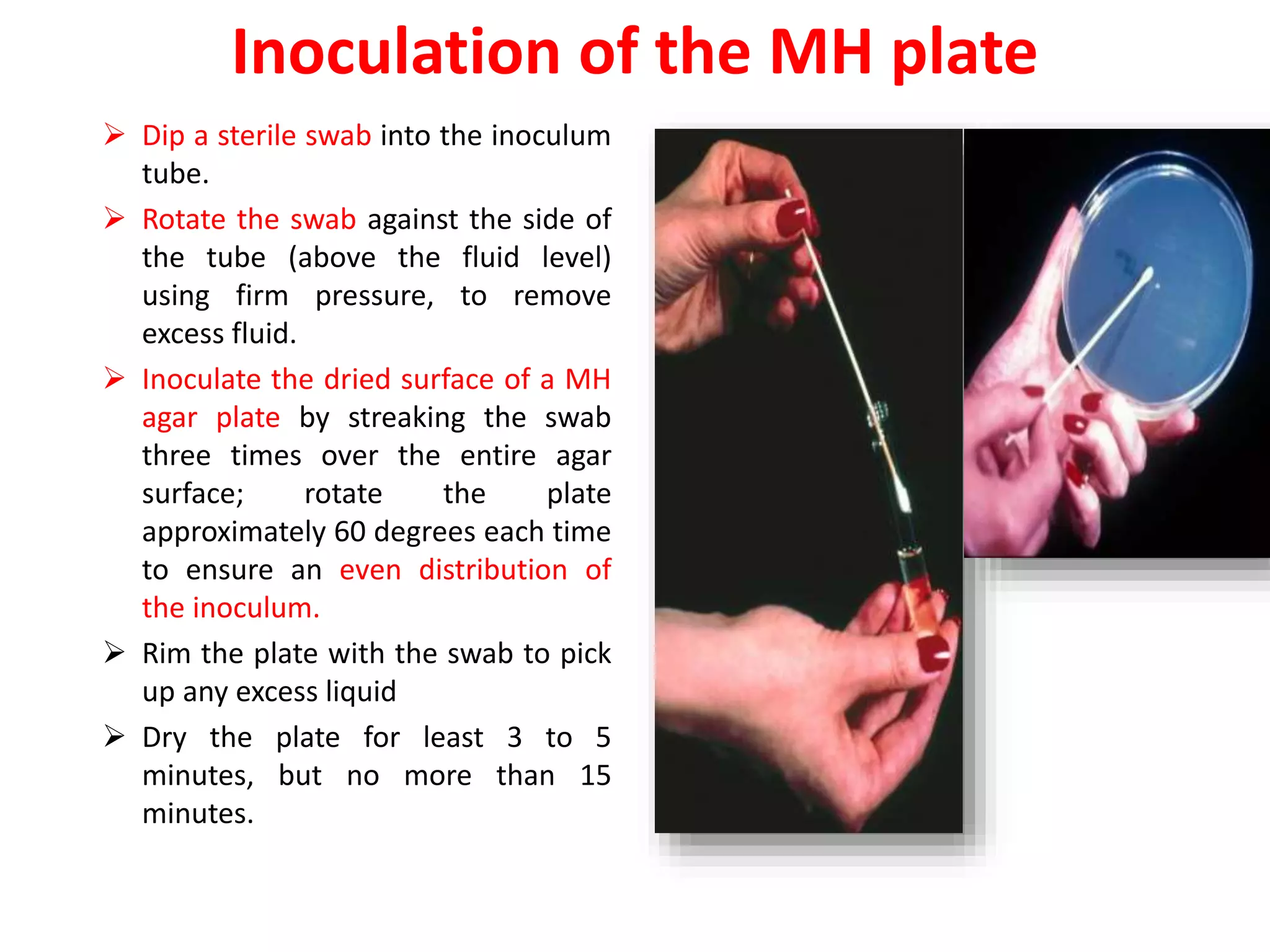
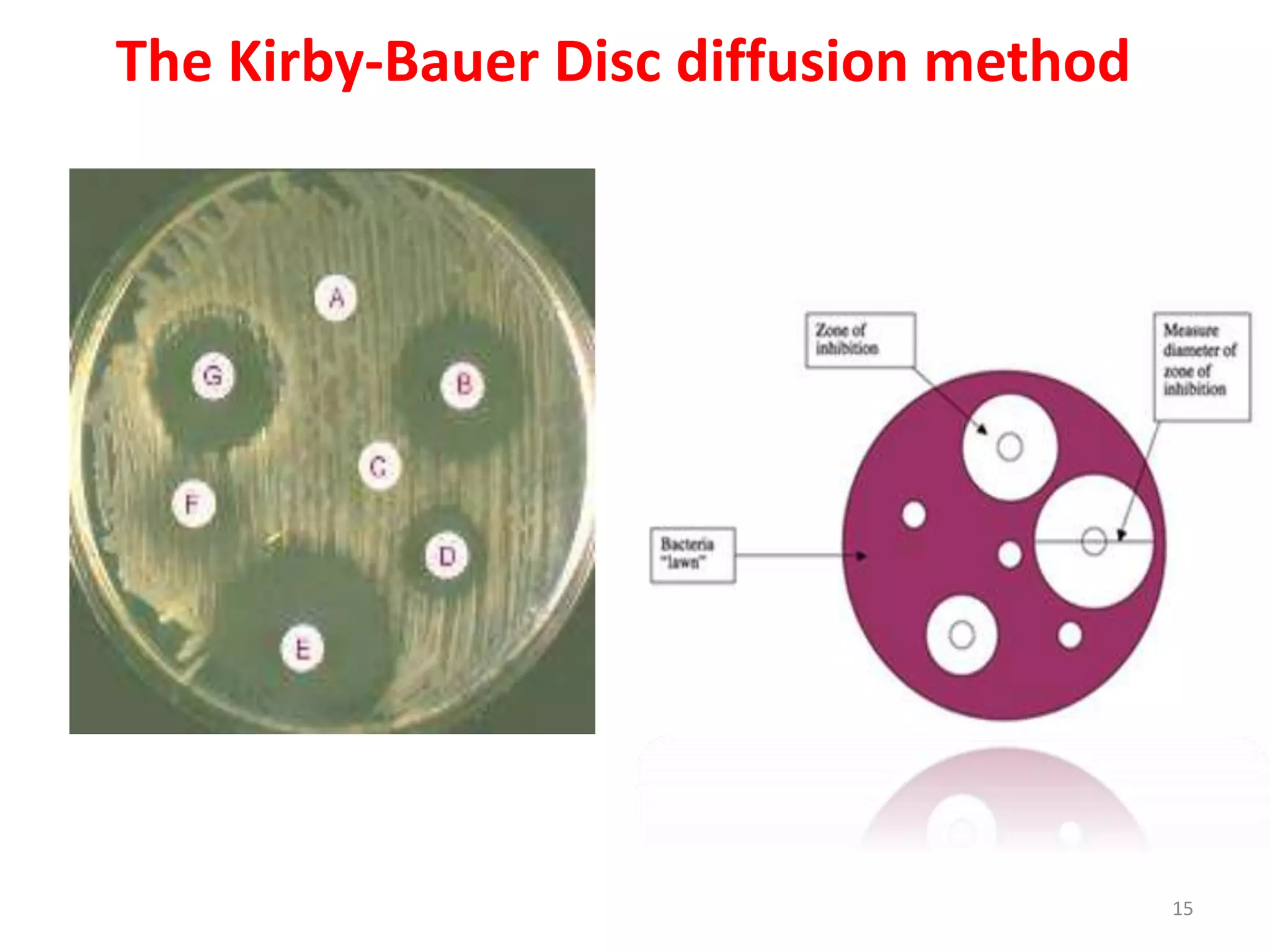
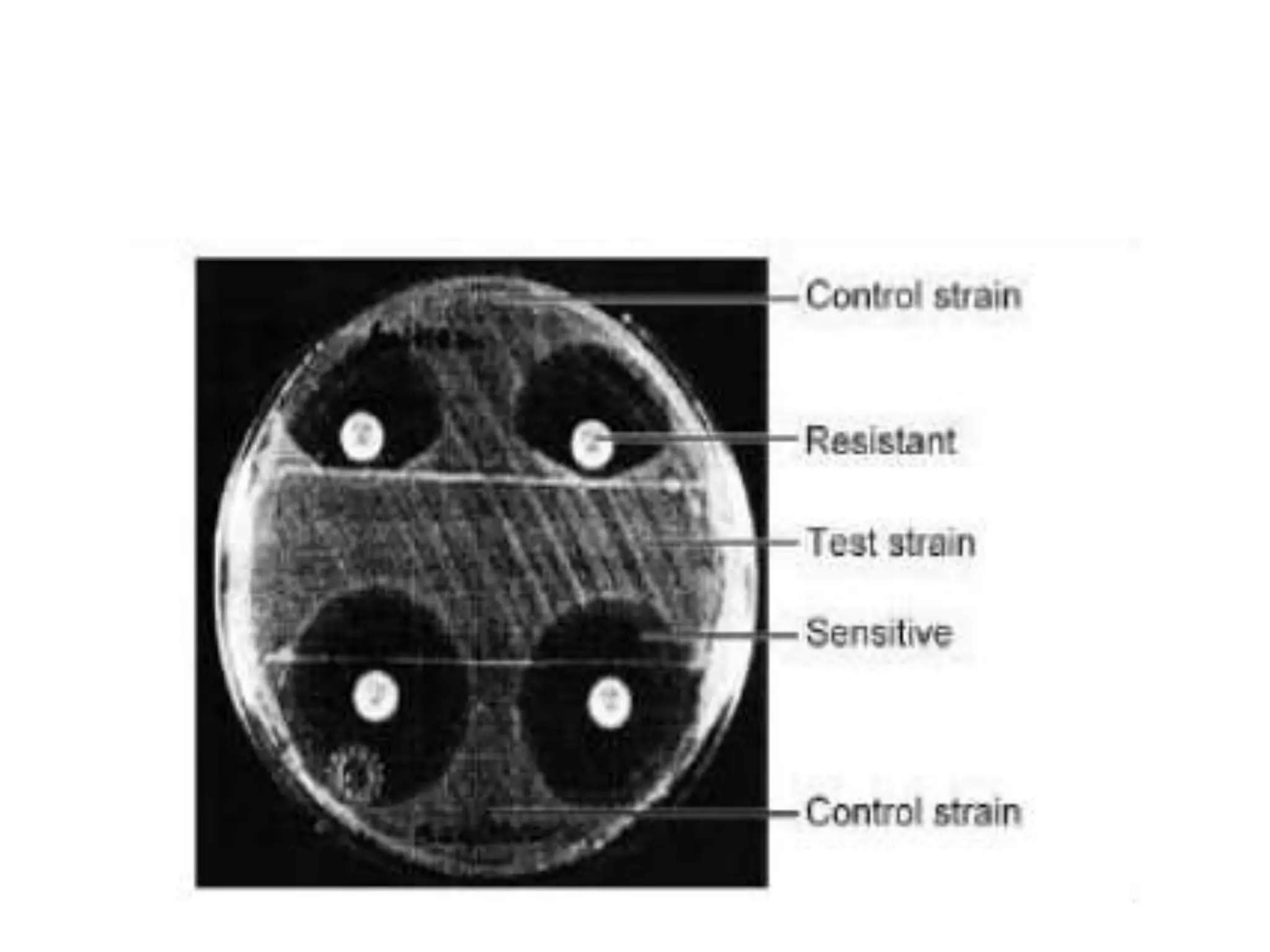
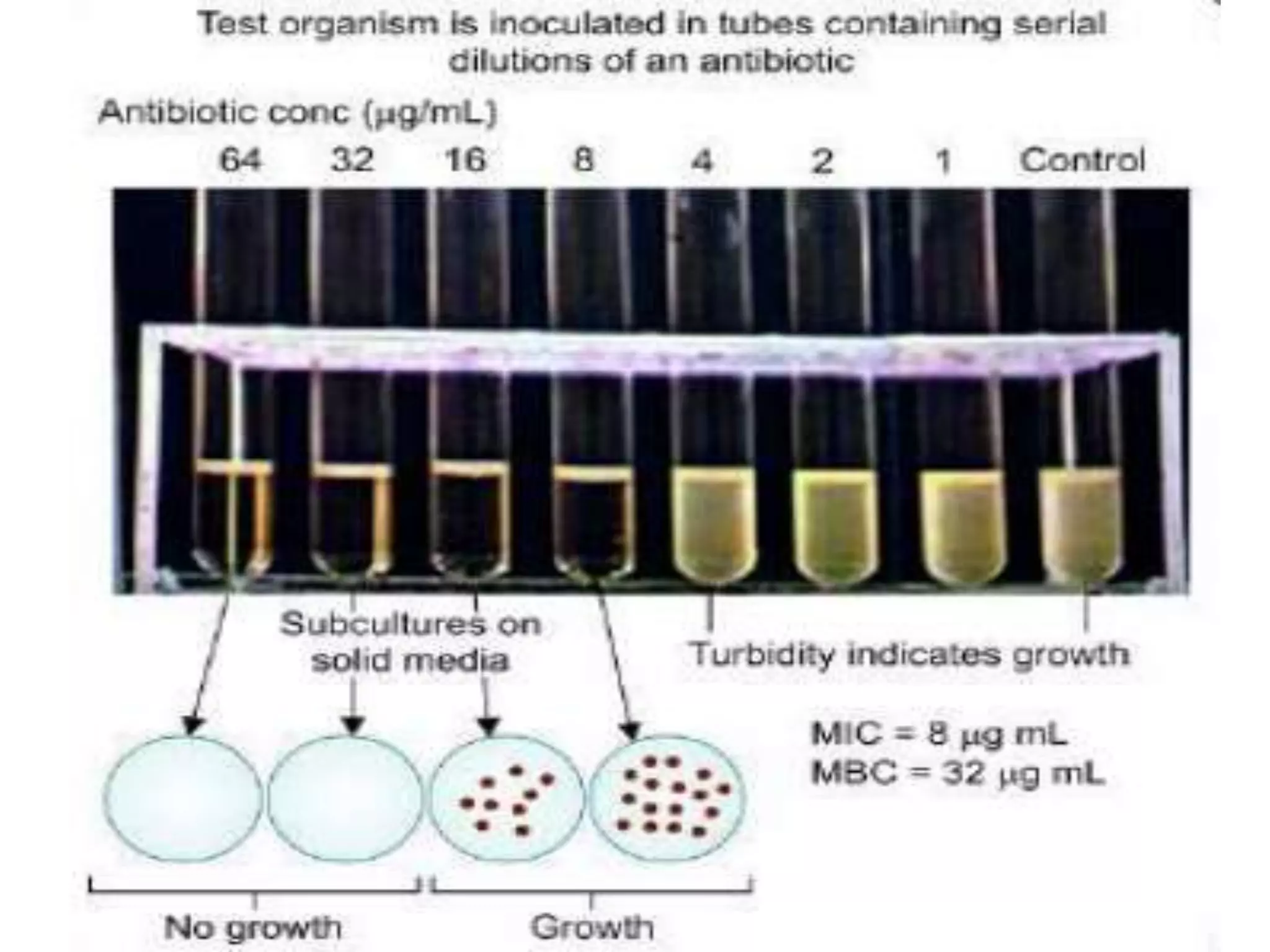
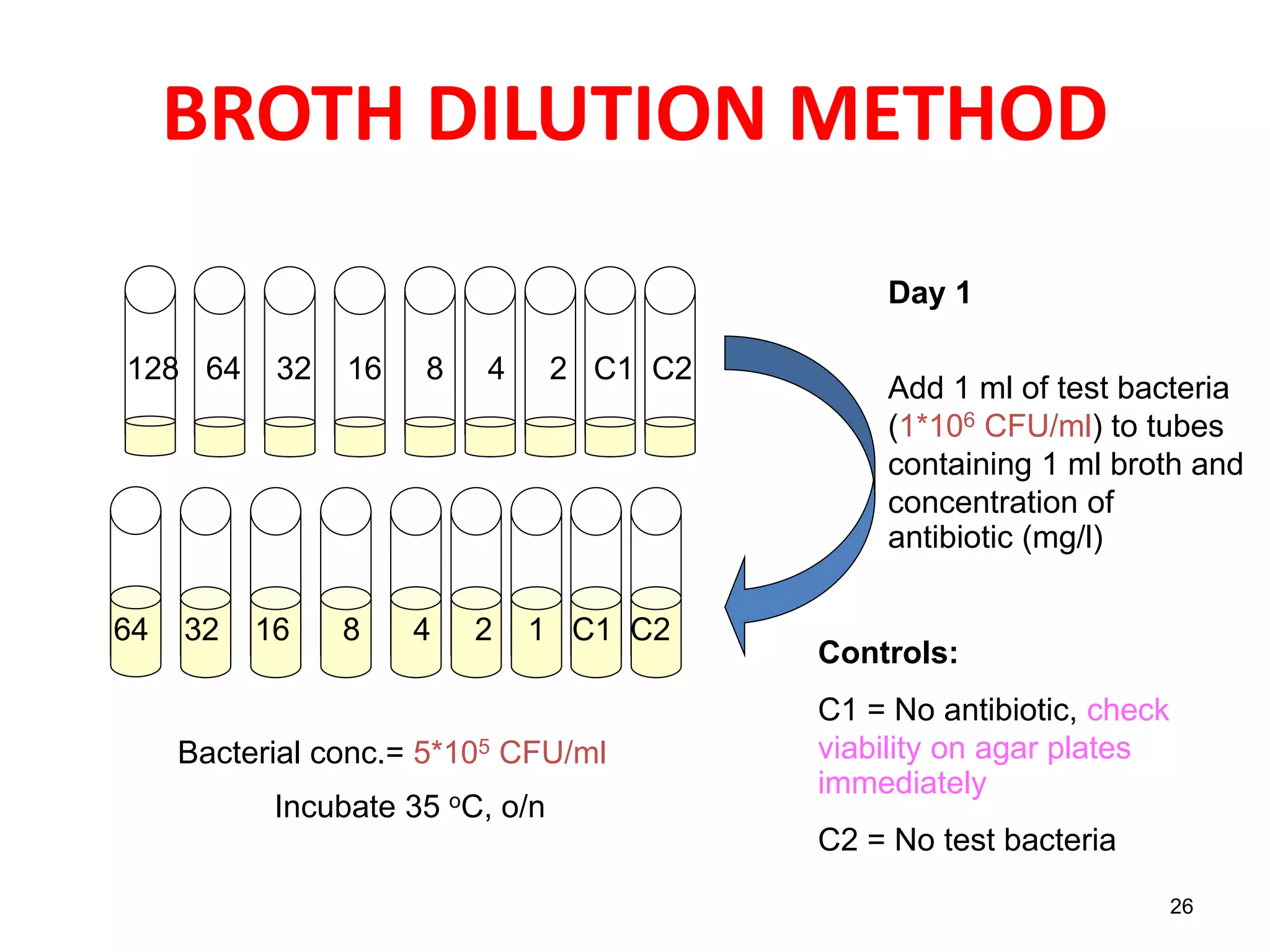
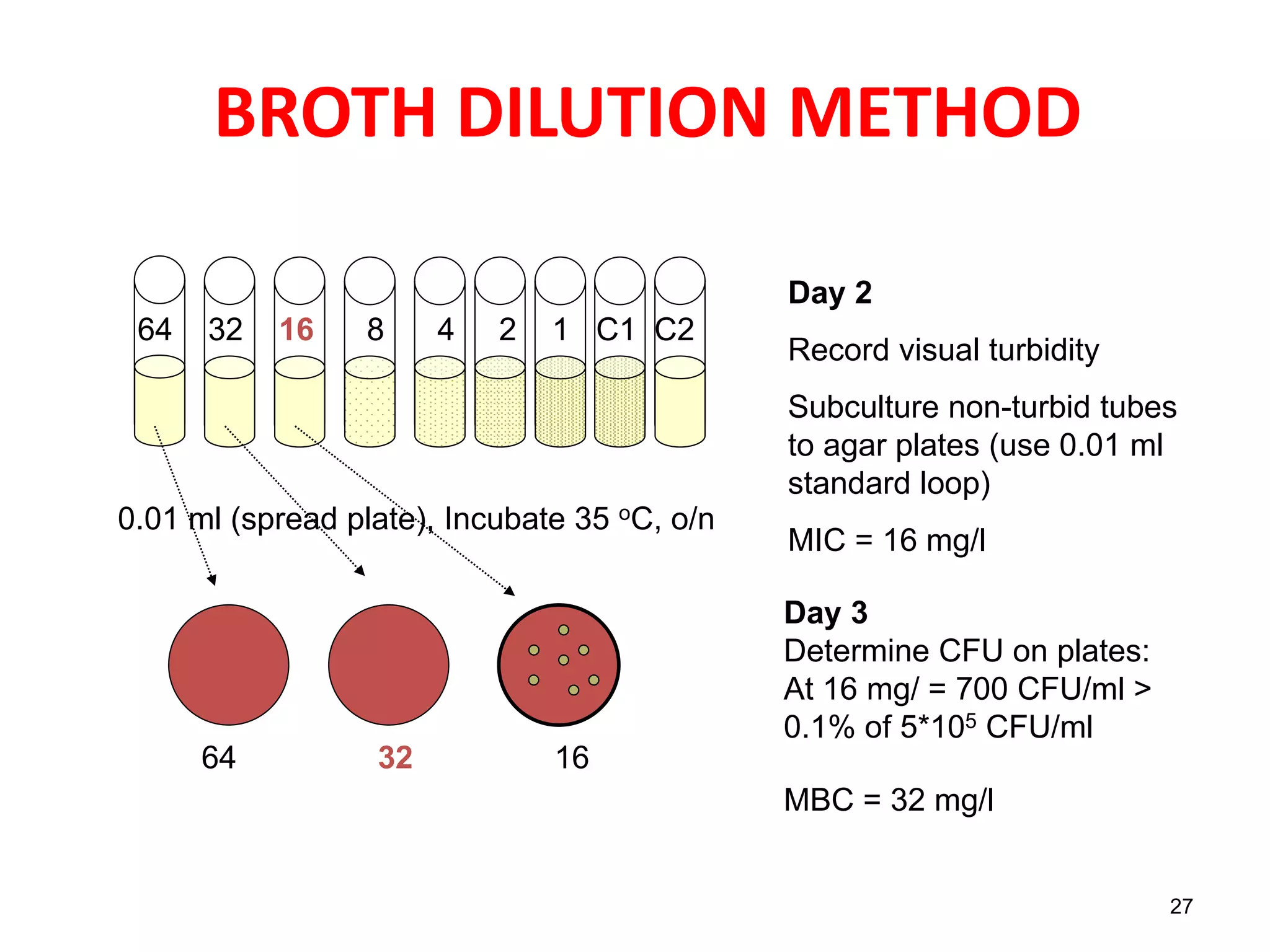
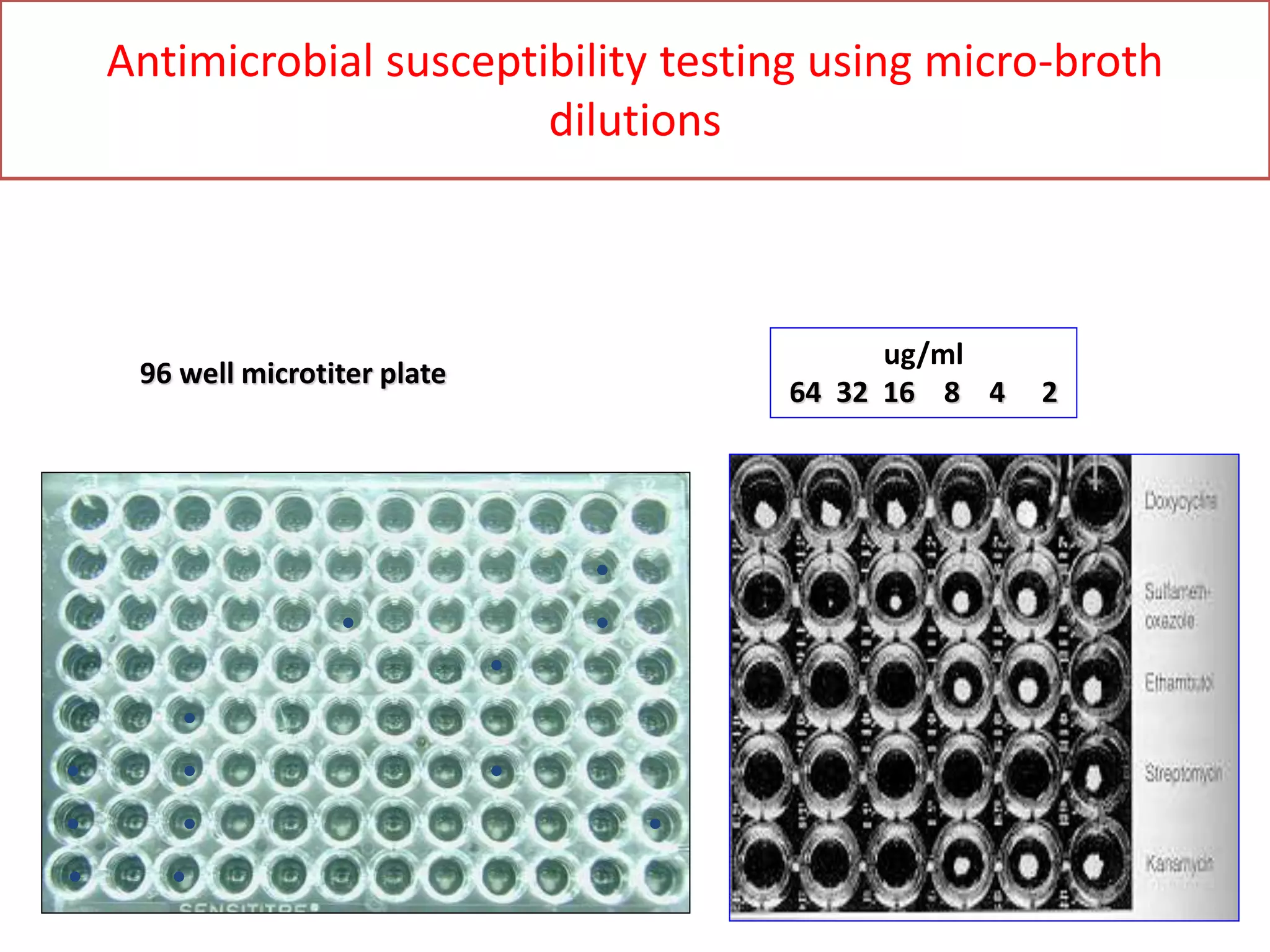
The document discusses antibiotic sensitivity testing (AST), which measures the susceptibility of bacterial isolates to different antibiotics. AST aims to select the most appropriate antibiotic for patients and assess emerging resistance patterns. Common AST methods include disk diffusion, broth dilution, Etest, and automated systems. Disk diffusion is the most widely used method and involves placing antimicrobial disks on agar plated with the test organism and measuring inhibition zone sizes. Broth dilution determines the minimum inhibitory concentration (MIC) by visually inspecting bacterial growth in serial antibiotic dilutions.