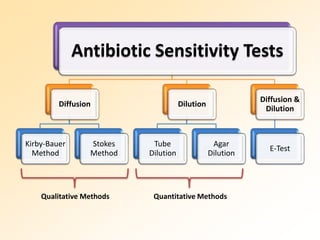
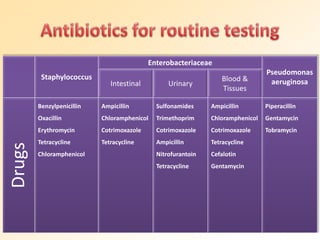
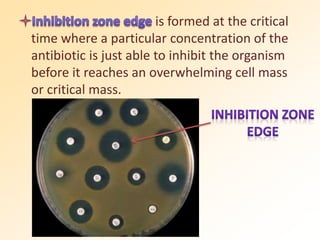
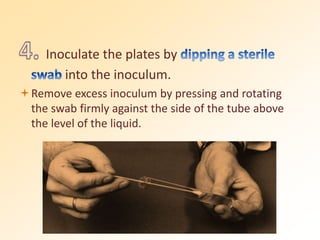
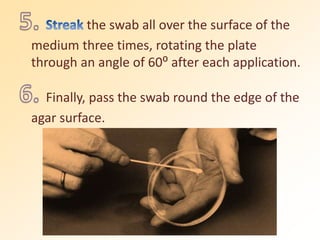
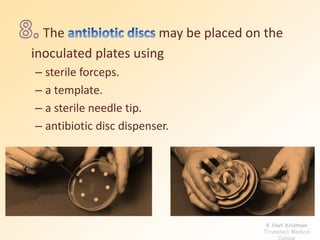
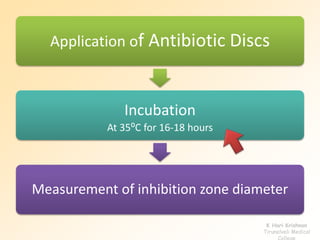
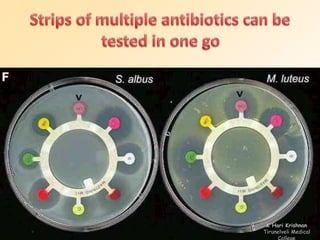
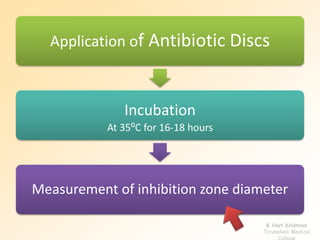
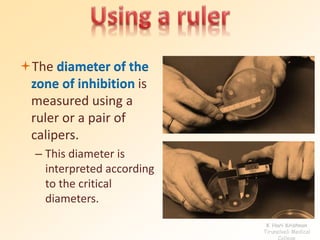
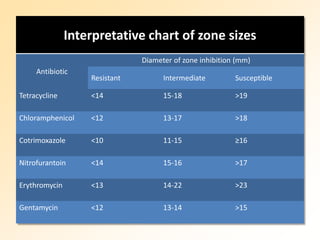
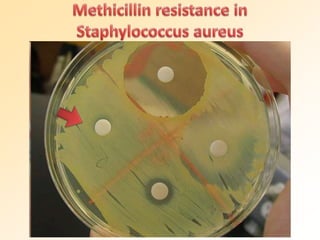
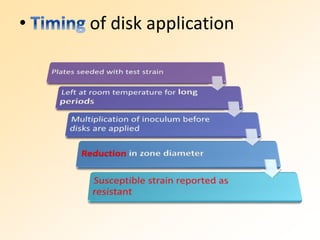
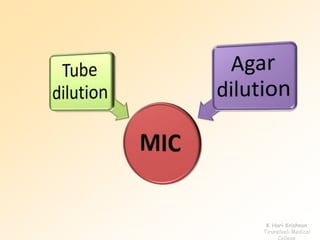
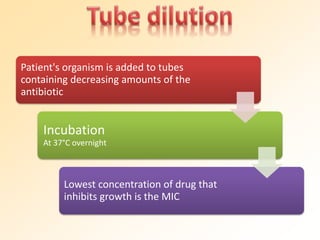
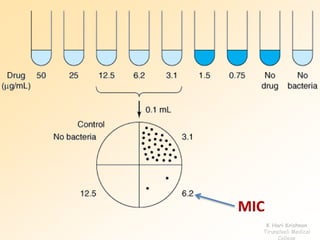
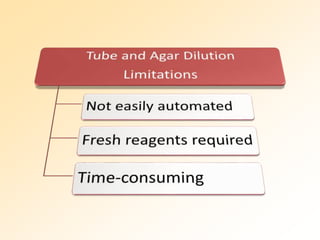
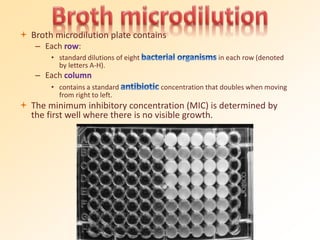
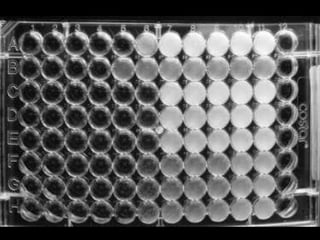
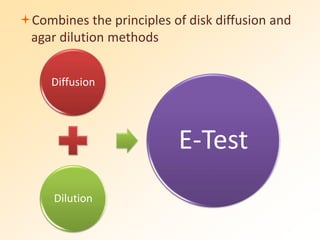
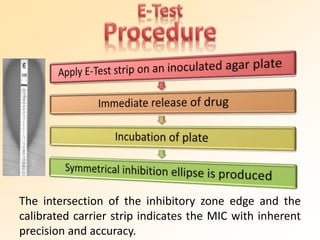
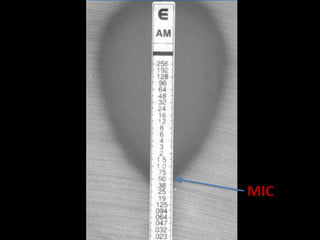
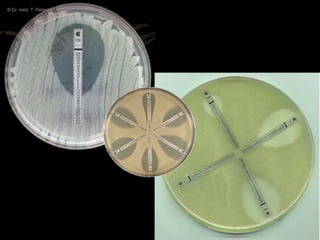
This document discusses antibiotic sensitivity testing (AST), which determines which antibiotics will effectively treat a bacterial infection. AST involves growing bacteria in culture with different antibiotics to see which ones inhibit growth. This helps clinicians select the most effective antibiotic for a patient. Common AST methods include disk diffusion, dilution tests, and automated systems. Care must be taken to standardize test conditions for reliable results. AST also provides epidemiological data on antibiotic resistance trends in a community.