The document provides an overview of antimicrobial susceptibility testing (AST) methods, including diffusion methods (like Kirby-Bauer), dilution methods (agar and broth), and automated systems, as well as methods for testing antiviral and antifungal susceptibility. It details the importance of AST in determining appropriate antibiotic therapy and assessing the effectiveness of treatments against bacterial, viral, and fungal infections. Additionally, it highlights the standardization efforts and references established for best practices in susceptibility testing.




![IMPORTANCE/GOALS OF AST :
• The identification of relevant antibiotics to specific pathogens in exudates and
body fluids collected from patients.
• Sensitivity tests down to determine the degree of sensitivity or resistance of
pathogens isolated from patient to an appropriate range of antimicrobial drugs .
• Assay of concentration of an administered drug in blood or body fluid of patient
required to control the schedule of dosage.
• The RESULTS of AST should be combined with clinical information and experience
when selecting the most appropriate antibiotic for patient .
• The raw data are either in form of a [INHIBITORY ZONE ] OR [MIC] . Typically , raw
data are interpreted and reported out as;](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-5-320.jpg)

![• THESE TESTS ARE PERFORED IN-VIVO AND UNDER STANDARDIZED
CONDITIONS:
1. STANDARD MEDIA : Muller-Hinton agar or broth.
2. STANDARD INOCULUM : Isolation and standardizing the bacteria suspend by
Macfarland standards [ Mcf =1.5microMF ].
• Macfarland standard : standard unit to measure turnbidity of bacterial suspension .
3. INCUBATION TIME AND TEMPERATURE : (35-37) ,16-18 hrs .
4. VOLUME OF MEDIA USED : 0.5 Mcf std.](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-7-320.jpg)
![TYPES OF ANTI-BACTERIAL
SUSCEPTIBILITY TESTING :
DILUTIO
N
DIFFUSION
AST
[DIFFUSION
AND
DILUTION]
AGAR DIUTION
BROTH DILUTION
STROKES DISC
DIFFUSION
KIRBY BAUER DISC
DIFFUSION
E-TEST](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-8-320.jpg)

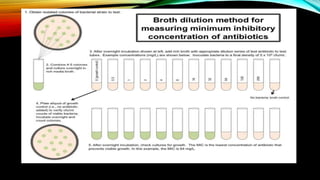
![• CONTROL : max growth (max. turbidity).
• As the concentration of antibiotics increases , the turbidity decreases .
• At a spwcific concentration – no turbidity -( MIC ).
• If the whole process sis done in petridish , it is called as MICROBROTH DILUTION
METHOD .
• ADVANTAGE : Quantitative method [MIC can be done ].
• DISADVANTAGE : 1) fastidious organisms can not be done .
2) Cumbersome procedure.](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-11-320.jpg)
![• AGAR DILUTION METHOD :
• MEDIUM : cation adjusted muller-hinton agar
• ANTIBIOTIC CONCENTRATION : 0.1-0.2 micro gm / ml
• INCUBATION TIME : 16-20 hrs ,(35-37 )celcius.
• PROCEDURE :
i. Pour the media in sterile petri plates .
ii. Prepare serial dilution of antibiotic concentration .
iii. Spread it through sterile glass spreader .
iv. Look for lowest concentration of antibiotic prevent the appearance of colonies
[MIC].
• ADVANTAGES: 1) fastidious bacteria can be tested
2) Can be used for anaerobes
3)MIC can be determined .
• DISADVANTAGES : 1) Cumbersome procedure .](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-12-320.jpg)



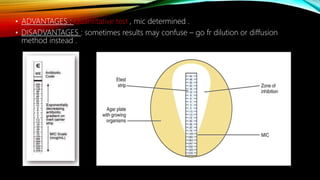





![• ADVANTAGES : 1) used to assess combined effect of multiple resistance mutation on
drug susceptibility .
2) Useful for assaying viruses [such as ; hepaitis B virus , HIV -1 , HCMV ]
• DISADVANTAGES : 1) labor intensive .
2) Expensive .
3) long exposure time / long turn around time .](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-25-320.jpg)
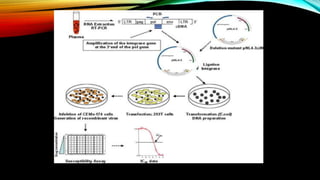

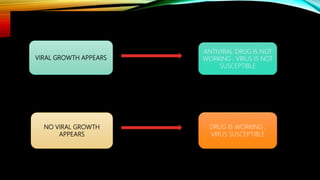


![PLAQUE REDUCTION ASSAY :
• Standard method of antiviral susceptibility assay .
• PRINCIPLE : determine of viral plaque formation in presence of antiviral agent .
• CONCENTRATION OF ANTIVIRAL DRUG :
• Inhibit plaque formation by 50% is [ IC 50].
• [50% inhibitory concentration +50% effective concentration ].](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-31-320.jpg)
![DYE UPTAKE ASSAY :
• Used for years .
• Determine viral cell lytic activity .
• Virus- in prescence of antiviral drug .
• Alive and viable cell.
• Takes vital cell [neutral red ] + HSV infection .
• RESULTS : dye bound to in comparison to dye bound to uninfected cell .
• This determines the extent of viral lytic activity .
• Drug concentration that inhibits viral lytic activity by 50% is IC50](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-32-320.jpg)

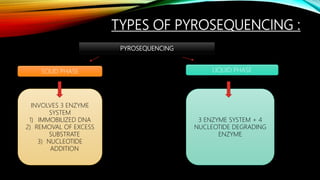
![PYROSEQUENCING :
• Most important method .
• Sequence based detection method .
• Allow rapid , accurate quantification of sequence variation .
• Allow rapid acquisition of short reads [100-200 bp ] of genomic sequence to
identify known mutation .
• Based on – technique of detection of released [Ppi] pyrpphosphate during DNA
synthesis .
• Visible light produced during reactions .
• Visible light strength generated directly proportional to number of nucleotide
incorporated into final product .](https://image.slidesharecdn.com/antimicrobialsusceptibilitytesting-210615194151/85/Antimicrobial-susceptibility-testing-34-320.jpg)