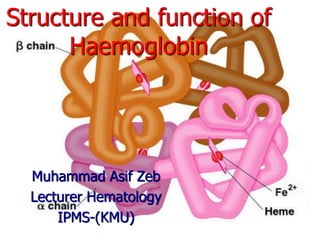
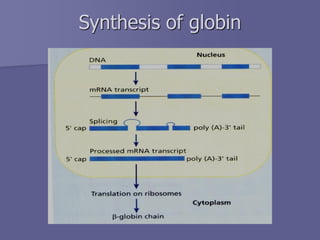
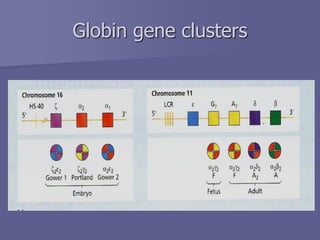
The document discusses the structure and function of hemoglobin, which is a vital protein in red blood cells responsible for oxygen transport. It details the synthesis of hemoglobin, including the production of heme and globin chains, and the various types of hemoglobin present at different life stages. Additionally, it covers how hemoglobin's ability to deliver oxygen is influenced by factors such as pH, carbon dioxide concentration, and the presence of 2,3-DPG.