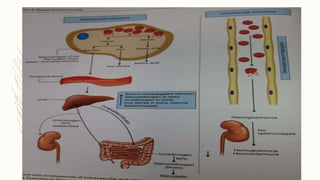
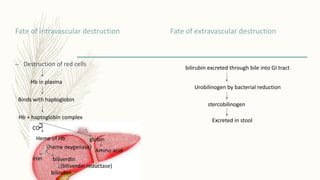
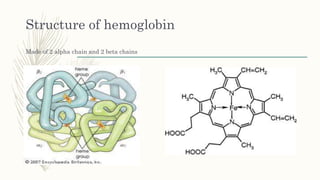
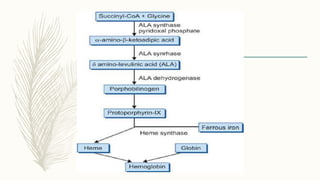
This document summarizes hemoglobin synthesis and functions. It discusses how hemoglobin is synthesized through the condensation of succinyl-CoA and glycine to form alpha-amino-beta-ketoadipic acid. It then describes the structure of hemoglobin as a globular molecule composed of four subunits, each with a polypeptide chain and heme. The document also outlines the main functions of hemoglobin, including transporting oxygen and carbon dioxide as well as acting as a buffer. It concludes by noting some physiological and pathological factors that can cause variations in hemoglobin concentration.