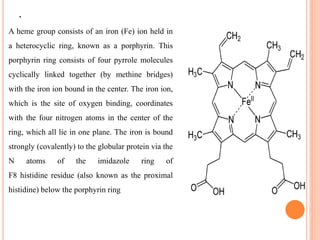
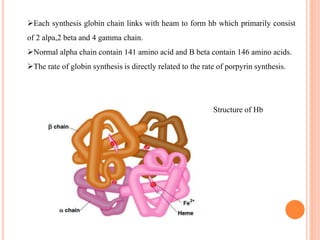
The document provides a comprehensive overview of hemoglobin, including its definition, structure, function, and detection methods. It details the synthesis process of hemoglobin, the different forms it can take, and abnormal variants along with their clinical significance. Additionally, various laboratory methods for measuring hemoglobin concentration, such as Sahli's method and cyanmethemoglobin, are described, highlighting the importance of hemoglobin levels in diagnosing conditions like anemia.