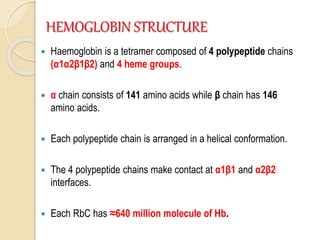
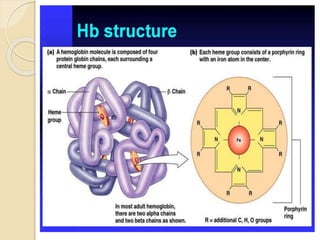
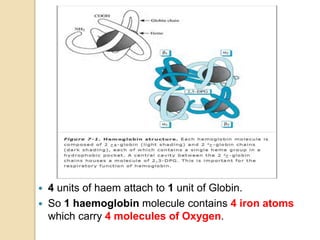
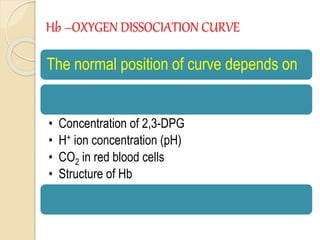
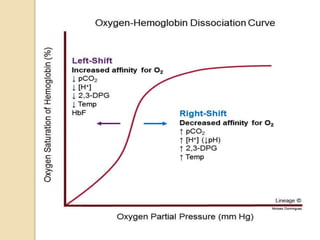
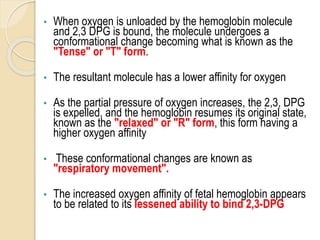
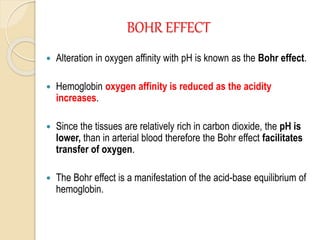
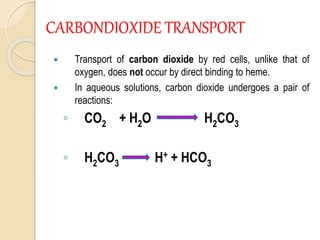
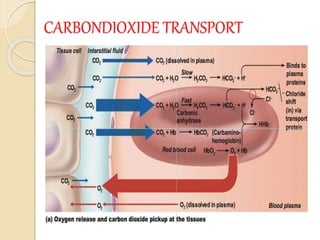
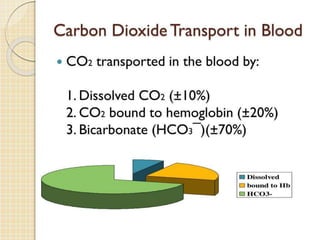
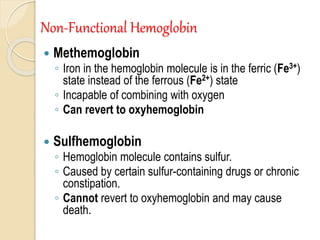
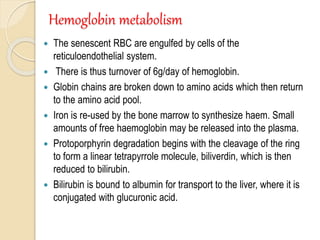
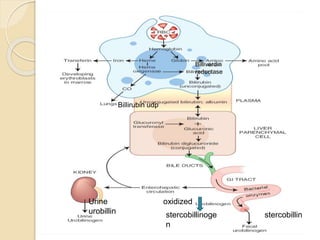
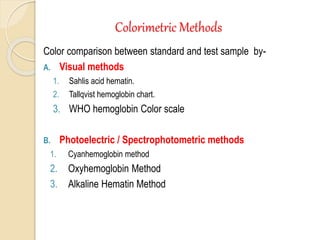
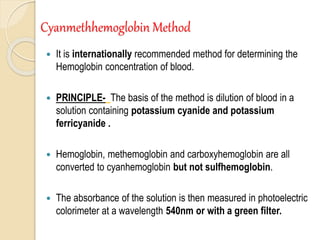
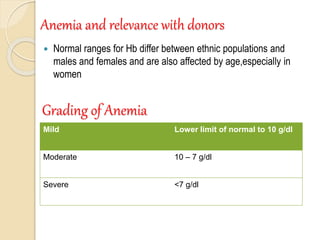
Hemoglobin is a protein in red blood cells that carries oxygen throughout the body. It is composed of heme and globin. There are over 500 hemoglobin variants but all have the same basic structure of four polypeptide chains, each with a heme group. Hemoglobin transports oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. The oxygen affinity of hemoglobin is affected by factors like pH, temperature, 2,3-DPG levels, and hemoglobin variants. Hemoglobin is broken down at the end of the red blood cell lifespan, with iron and amino acids being recycled and heme being broken down to bilirubin and excreted.