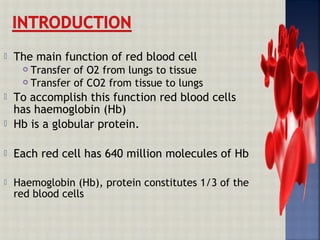
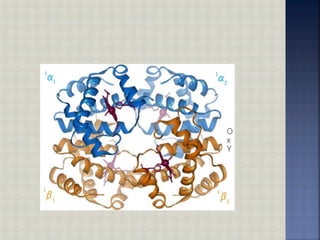
Hemoglobin is an iron-containing protein in red blood cells that transports oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. It is a globular tetrameric protein composed of two alpha and two beta chains, with each chain containing a heme group that binds to oxygen. Hemoglobin undergoes a conformational change upon oxygen binding that makes the remaining binding sites have a higher affinity for oxygen in a cooperative binding process essential for oxygen transport.
![Haem & globin produced at two different sites
in the cells
Haem in mitochondria
Globin in polyribosomes
Haemoglobin is involved in transport of other
gases such as co2 carboamino haemoglobin {co2 is
bound to globular protein.}
Protein found in root nodules of nitrogen fixing
root nodules [leguminous plants]Legheamoglobin](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-4-320.jpg)
![ Haemoglobin [Hb or Hgb] is the iron containing
oxygen transport metalloprotein in red blood
cells of all vertebrates.
Structure was elucidated by Max Preutz {Father
of X-ray crystallographic method}
Hb A is Haemoglobin found excluseivly in RBC’s
of adults,and is composed of four polypeptide
chains.
It’s a terameric protein with quaternary
structure
––{ two sets of similar units ({ two sets of similar units (αα22ββ22)})}](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-5-320.jpg)







![ Heme contains:
a) organic part protoporyphirin
b) Inorganic part Fe atom [metal]
Four Pyrrole groups [A to D] linked byFour Pyrrole groups [A to D] linked by
methane bridgesmethane bridges
Ferrous in heme acts as prosthetic group
Ferrous [Fe2+] ion resides at the center of
tetrapyrole ring called hydrophobic heme
pocket.
Hemoglobin has 4 heme,hence can carry 4
oxygen atoms.](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-13-320.jpg)

![A molecule of OA molecule of O22 acts as 6acts as 6thth
ligand.ligand.
Porphyrin rings in heme with its particularPorphyrin rings in heme with its particular
arrangement of 4 methyl, 2 propionates & 2arrangement of 4 methyl, 2 propionates & 2
vinyl substituentsvinyl substituents protoporyphyrin I Xprotoporyphyrin I X
In deoxy form,theIn deoxy form,the ferrous atoms sit out of planeferrous atoms sit out of plane
poryphyrinporyphyrin by about 0.6 Angstrom.by about 0.6 Angstrom.
This is due to stearic repulsion between HisThis is due to stearic repulsion between His
F8[proximal histidine] and the poryphyrin plane.F8[proximal histidine] and the poryphyrin plane.
When oWhen o22 binds ,staeric repulsion is minimisedbinds ,staeric repulsion is minimised
and ferrous atom comes to the plane.and ferrous atom comes to the plane.
Oxygenation changes electrostatic state of FeOxygenation changes electrostatic state of Fe2+2+
of heme.of heme.](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-15-320.jpg)




![PERUTZ MECHANISMPERUTZ MECHANISM
Hb has two conformational statesHb has two conformational states -- the deoxy orthe deoxy or TT
statestate andand the oxy orthe oxy or R state.R state.
In deoxy haemoglobin quaternary structureIn deoxy haemoglobin quaternary structure
interactions are constrainedinteractions are constrained T-stateT-state [Tensed[Tensed
state taut]state taut]
When OWhen O22 binds, it relaxes the quaternary structurebinds, it relaxes the quaternary structure
R-StateR-State [Relaxed state][Relaxed state]
[Relaxed coformation due to interaction][Relaxed coformation due to interaction]](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-20-320.jpg)








![ Left to Right binding of oxygen
1st
moulecule bound causes structural changes
that influences the binding of next molecule.
Initial slope is low as Hb is independently
competing for first o2 .
Once Oxygen molecule is bound to 1 Hb’s
subunits,increases the o2 binding affinity of its
other subunits
[thereby incresing slope of midle portion]](https://image.slidesharecdn.com/haemoglobin-160424181218/85/Haemoglobin-30-320.jpg)


