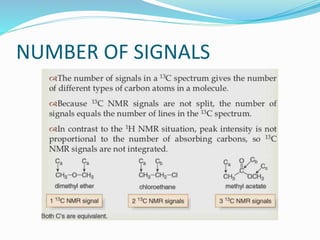
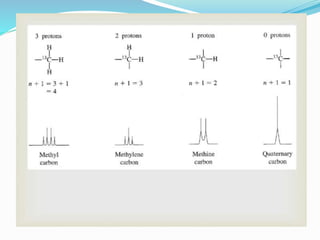
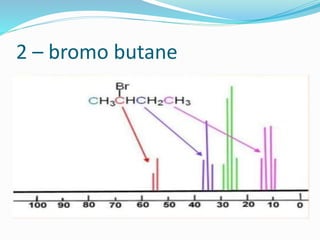
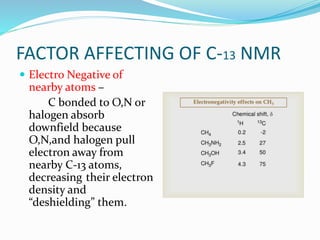
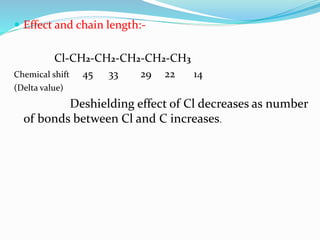
This document provides an overview of C-13 NMR spectroscopy. It discusses the history and principle of NMR spectroscopy, focusing on C-13. Key points include: C-13 has a nuclear spin of 1/2, allowing it to be detected by NMR, unlike C-12. The chemical shift range for C-13 is much broader than for proton NMR, from 0-220 ppm. The number of C-13 signals indicates the number of non-equivalent carbon types in a molecule. C-13 coupling is observed with directly bonded protons and other nearby nuclei. Applications of C-13 NMR include structure elucidation of organic and biochemical compounds.