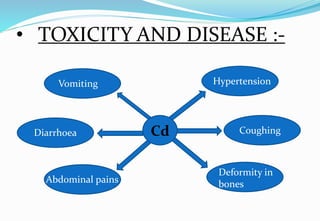
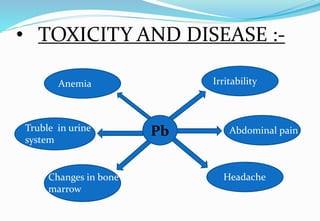
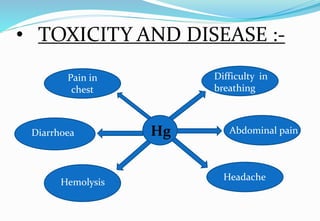
Heavy metal pollution from sources like industrial effluents and vehicle emissions is a growing global problem. Some key heavy metals that are toxic to humans include cadmium, lead, and mercury. Cadmium toxicity can cause vomiting, diarrhea and bone deformities. Lead exposure is linked to anemia, kidney problems, and changes in bone marrow. Mercury poisoning results in issues like diarrhea, breathing difficulties, and abdominal pain. Heavy metals are a threat because unlike some other pollutants, they do not break down in the environment.