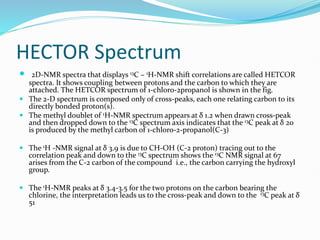
This document provides an overview of nuclear magnetic resonance spectroscopy using carbon-13 (13C NMR). It discusses key aspects of 13C NMR including the properties of 13C nuclei, chemical shifts, hydrogen decoupling, DEPT experiments, 2D NMR techniques like COSY and HECTOR, and applications of 13C NMR such as structure elucidation and in vivo analysis. Examples are provided to illustrate concepts like hydrogen decoupling, DEPT spectra, and 2D NMR correlations. In summary, the document serves as an introduction to 13C NMR spectroscopy, covering fundamental principles, experimental techniques, and applications of the method.