The Gattermann-Koch reaction is a chemical reaction discovered in 1897 by German chemists Ludwig Gattermann and Julius Arnold Koch. It involves the formylation (addition of an aldehyde group) of aromatic compounds using a mixture of carbon monoxide, hydrogen chloride, and anhydrous aluminum chloride catalyst. The unstable formyl chloride intermediate reacts to add the formyl group to the aromatic ring, producing an aromatic aldehyde such as benzaldehyde from benzene.






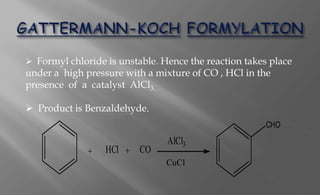
![The CO and HCl reacts to form an unstable formyl
chloride.
CO + HCl + AlCl3 → [ HC+ = O ↔ HC ≡ O+ ] AlCl-
4](https://image.slidesharecdn.com/gattermannkoch-200229164840/85/Gattermann-koch-8-320.jpg)




