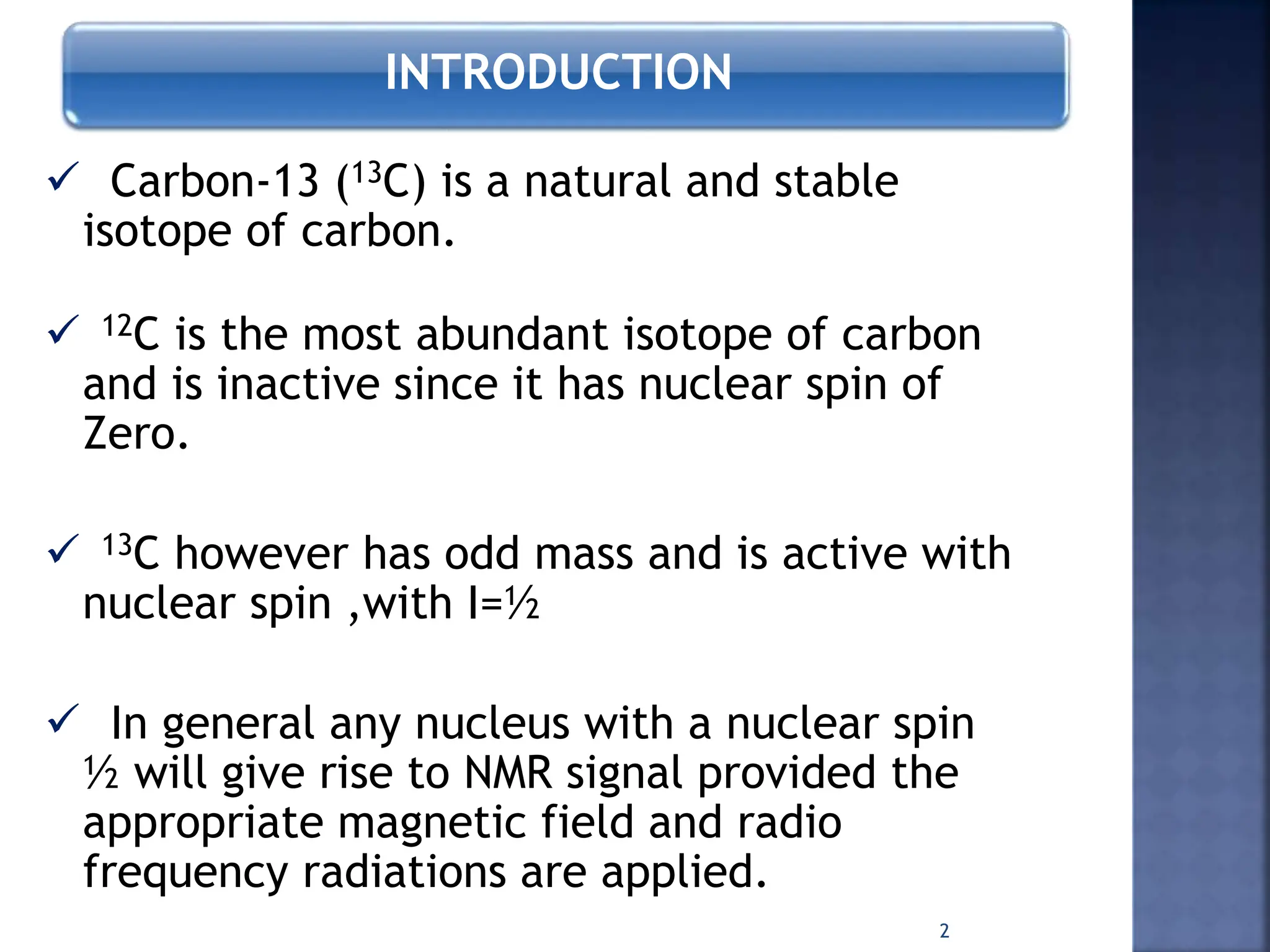
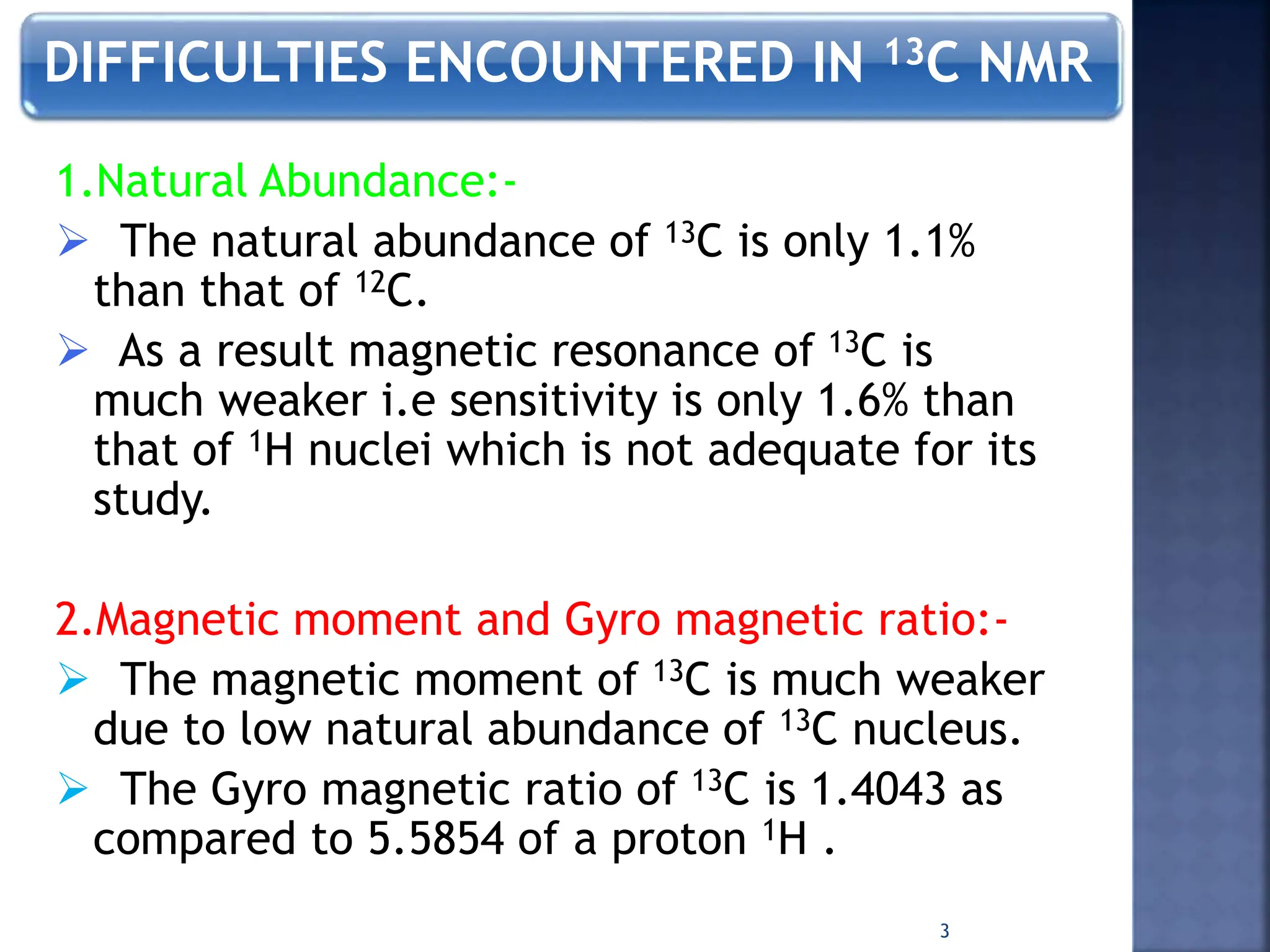
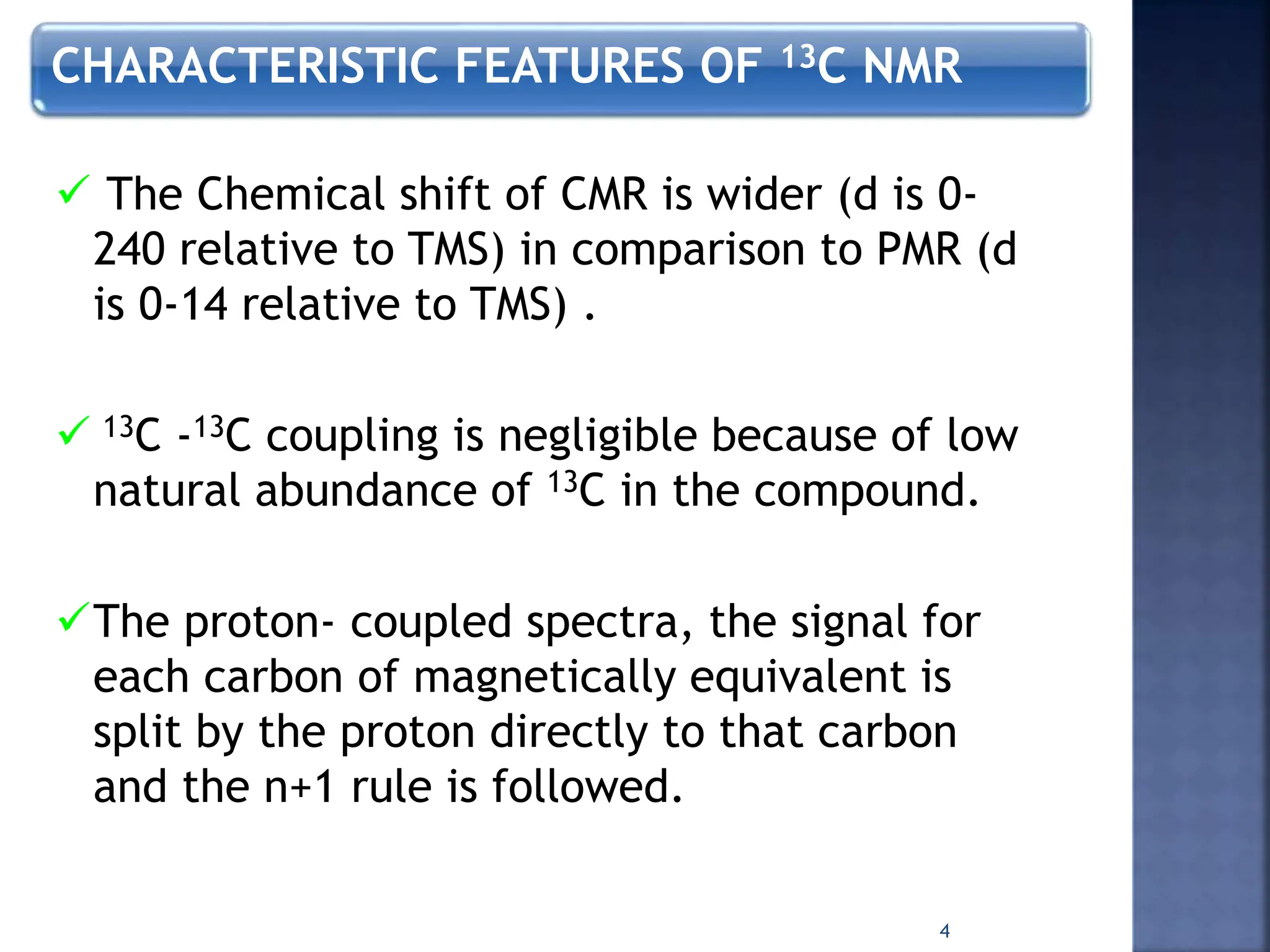
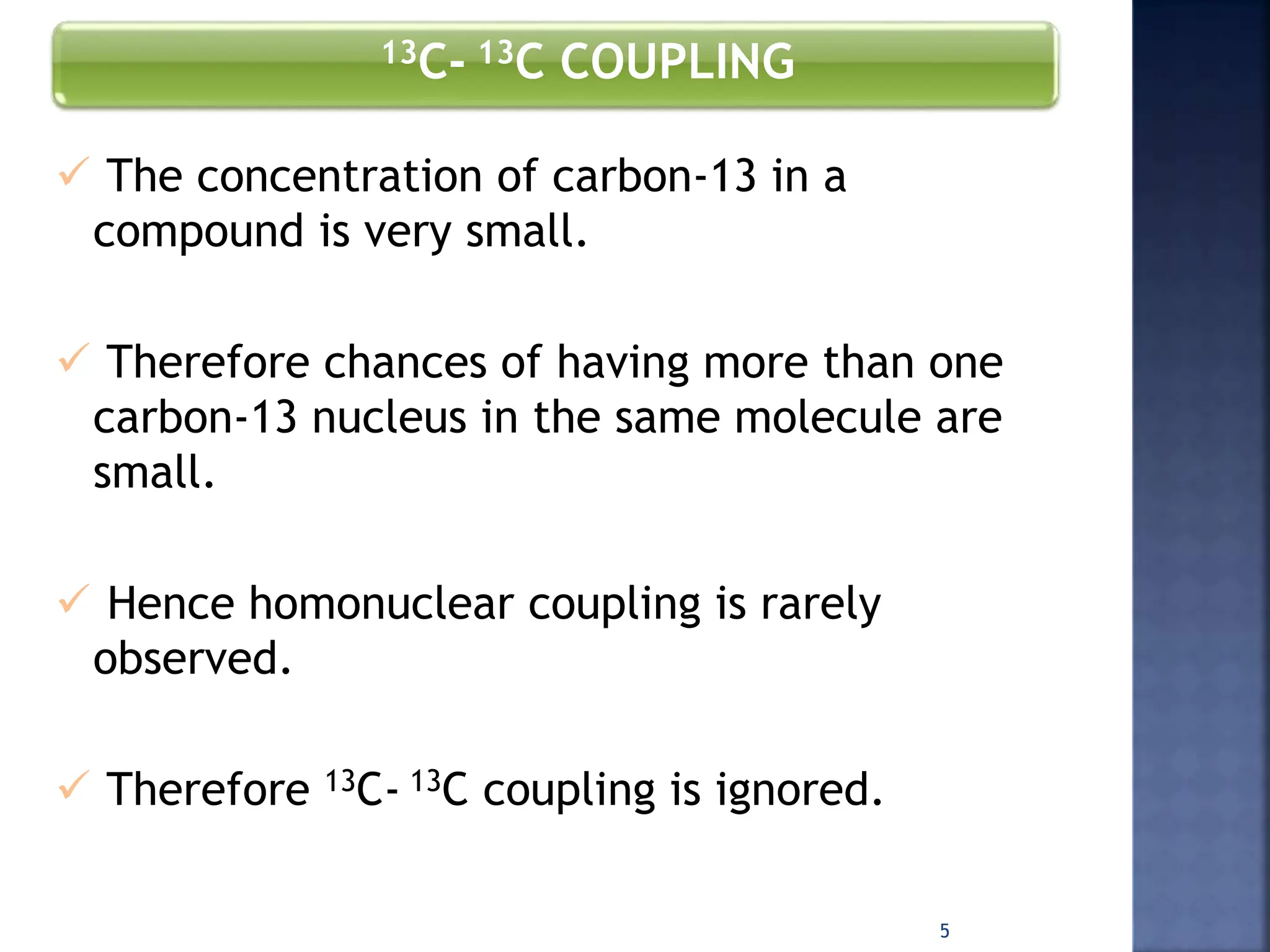
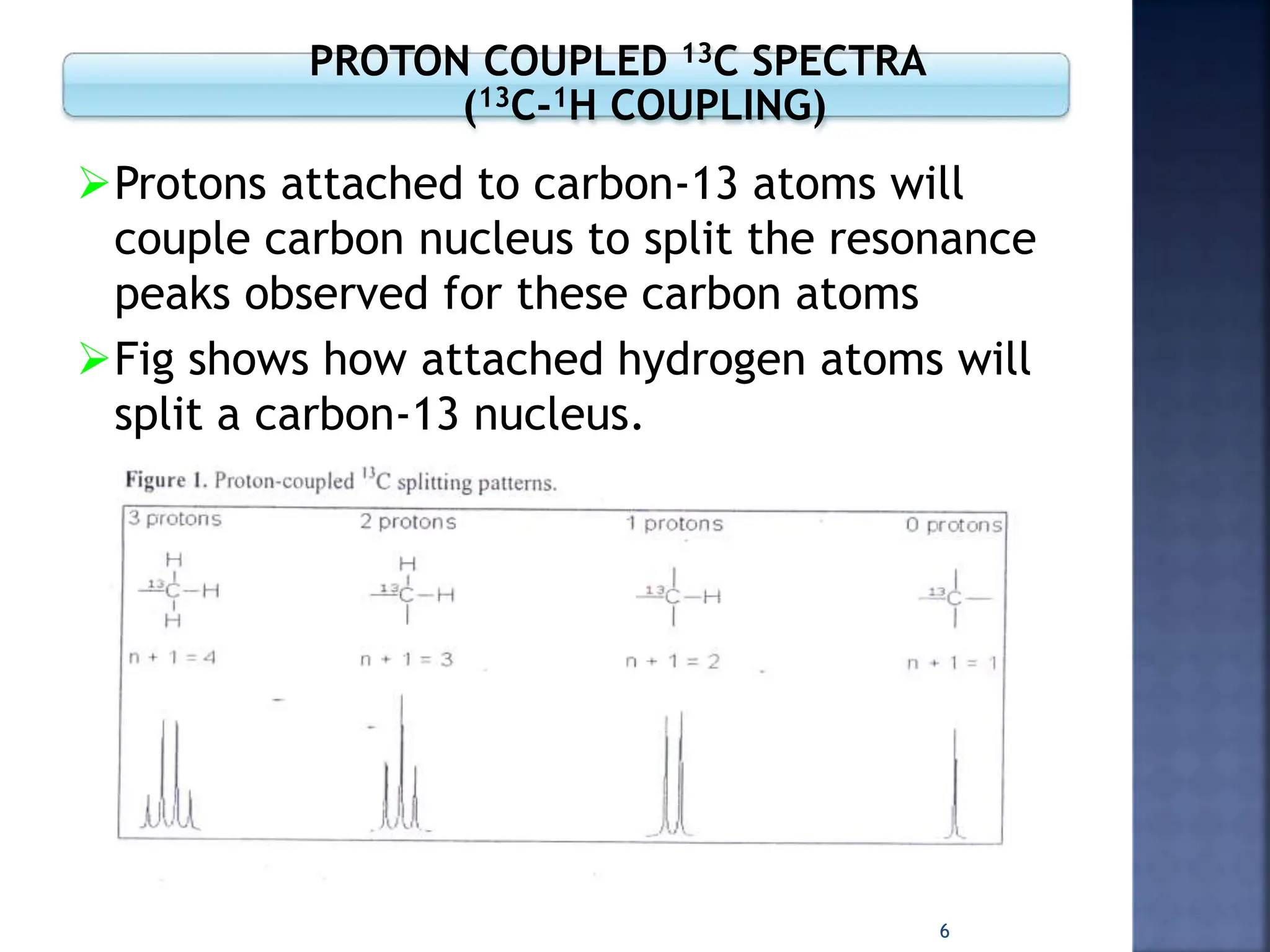
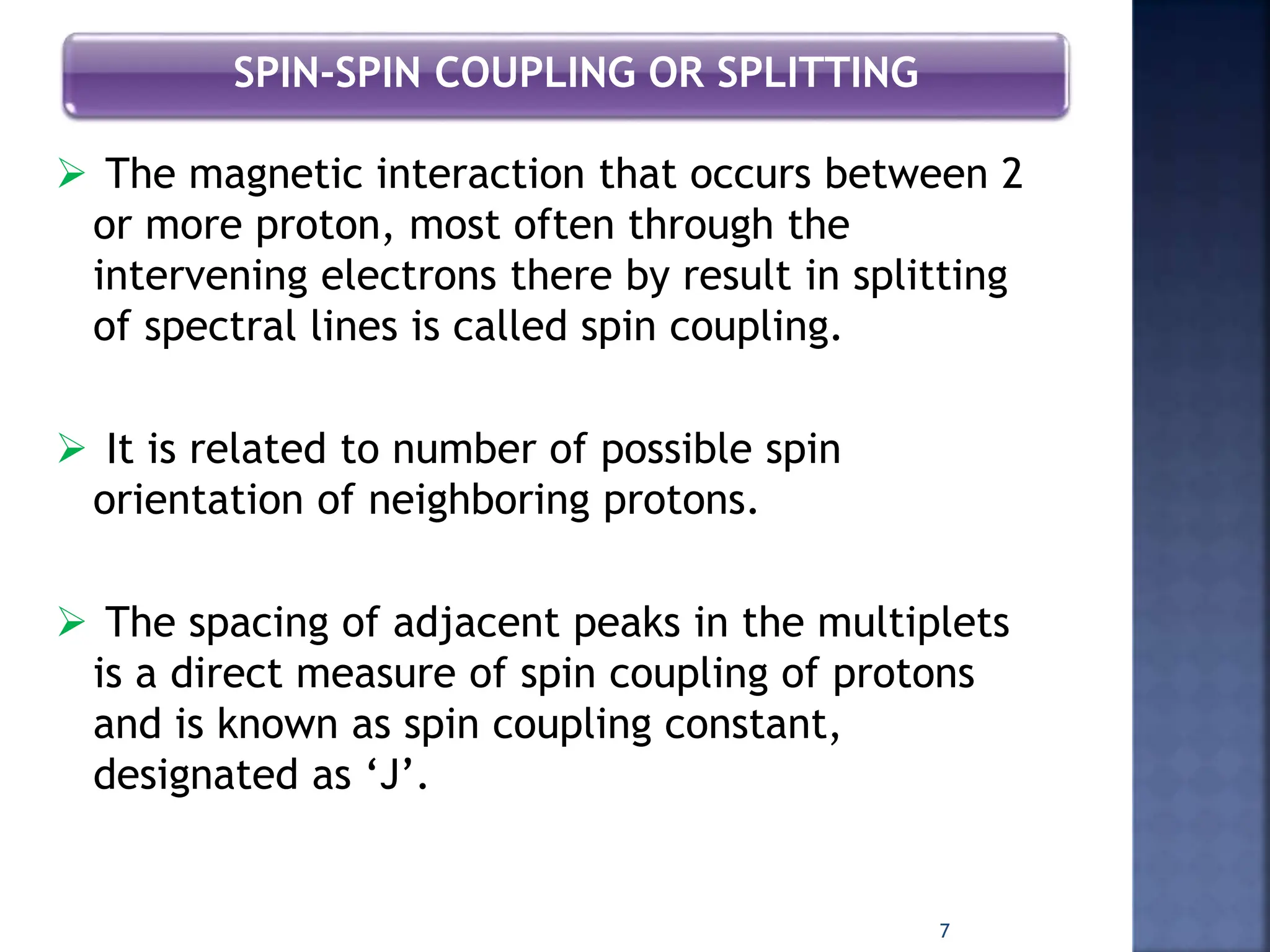
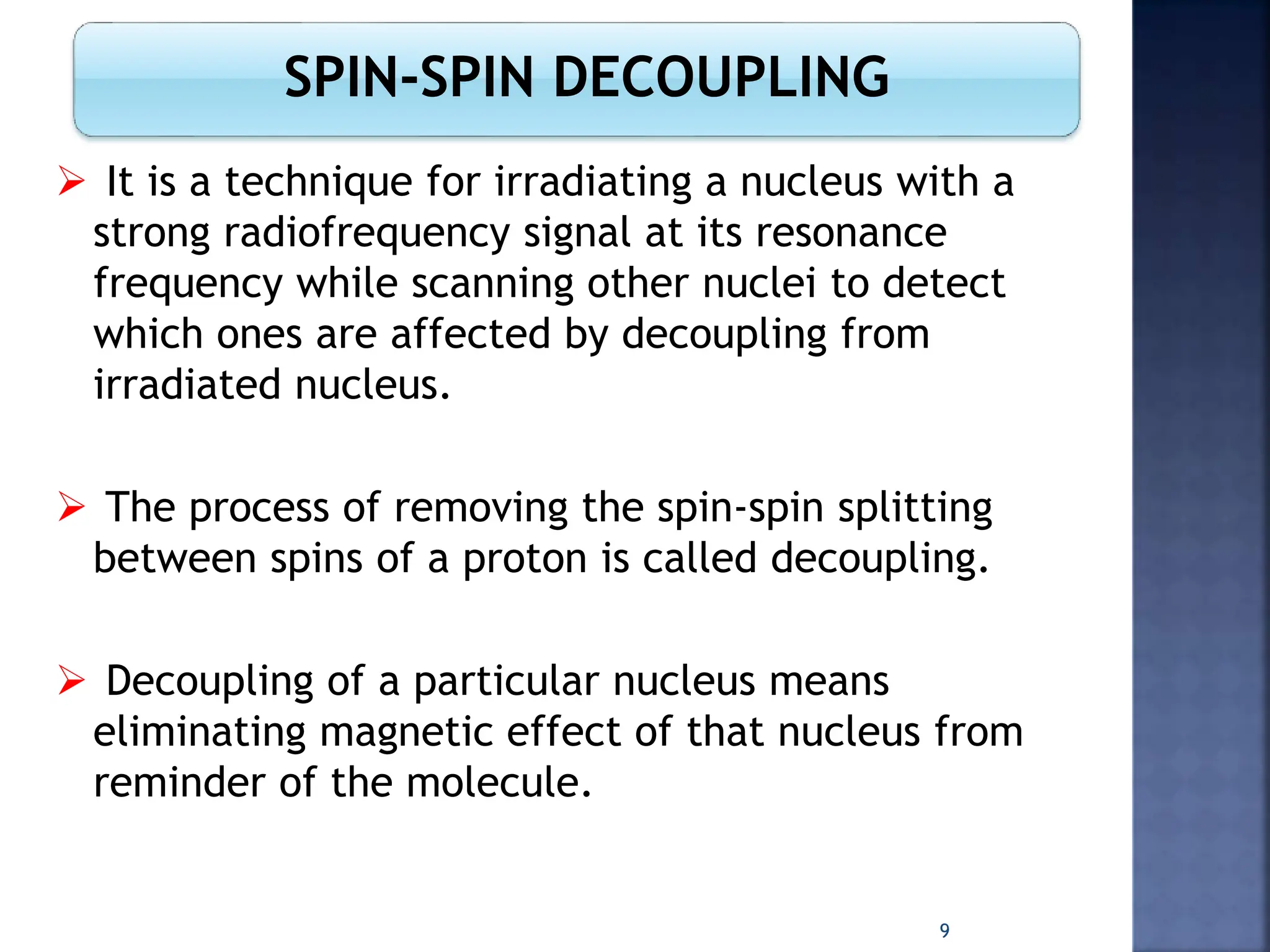
This document provides an overview of carbon-13 (13C) nuclear magnetic resonance (NMR) spectroscopy. It discusses the characteristics of 13C, including its low natural abundance and magnetic moment. It also describes the difficulties in 13C NMR spectroscopy related to sensitivity. The document outlines the features of 13C NMR spectra, such as chemical shift range and lack of 13C-13C coupling. Additionally, it explains proton-coupled 13C NMR spectroscopy and techniques to simplify complex spectra, such as decoupling, higher magnetic fields, and chemical shift referencing.

![TYPES OF DECOUPLING
Homonuclear decoupling
Heteronuclear decoupling
Homonuclear decoupling :- It is a selective
decoupling where the type of nucleus being
decoupled is same as that being observed.
Ex:- Decoupling one proton while observing other
proton.1H[1H]
Heteronuclear decoupling:-It is occurs when
decoupled nuclei are different from the observed
one.
Ex:- Decoupling of 1H while observing 13C. 13C[1H]
11](https://image.slidesharecdn.com/nmr-240216162323-830843af/75/Nuclear-magnetic-resonance-effect-introduction-principles-applications-11-2048.jpg)