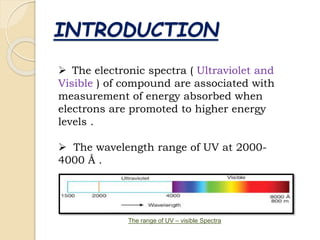
This document discusses ultraviolet-visible spectroscopy (UV-Vis). It begins with an introduction to UV-Vis spectroscopy and its history. The principle behind UV-Vis is then explained, noting that electrons are promoted to higher energy levels when absorbing ultraviolet or visible light. The document outlines the instrumentation used, known as a spectrophotometer, and Beer's Law which describes the relationship between absorbance and concentration. UV-Vis spectroscopy has various applications including quantitative analysis and identification of unknown compounds by comparing their spectra. The document concludes that UV-Vis is widely used in analytical chemistry.