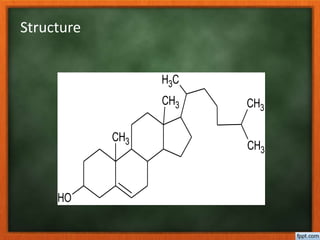
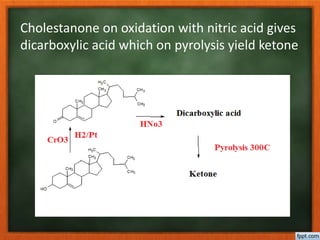
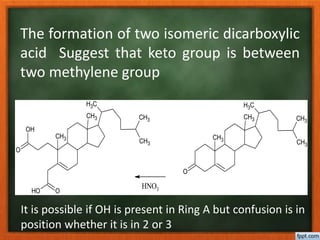
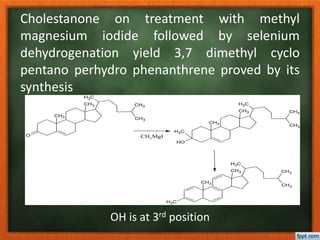
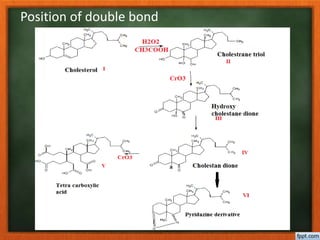
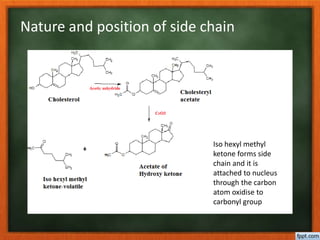
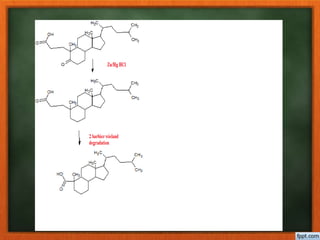
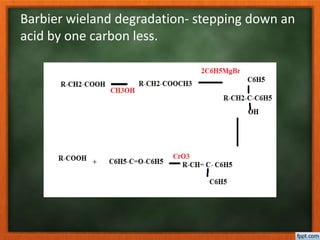
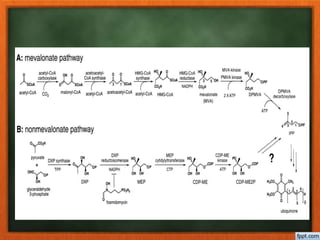
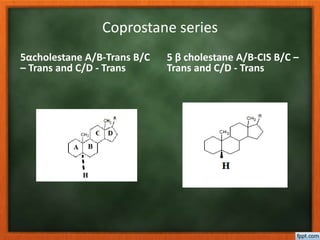
Cholesterol is present in all mammalian tissues, either in a free state or esterified with fatty acids. It is synthesized in the microsomal and cytosolic fractions of cells and is present in large quantities in brain and nerve tissue. Cholesterol has an optically active levo-rotatory structure and is an animal sterol that occurs as a free alcohol and in fatty acid esters. It was first isolated from human gallstones and deposits in the bile duct. The structure of cholesterol was determined through a series of chemical reactions and degradations.