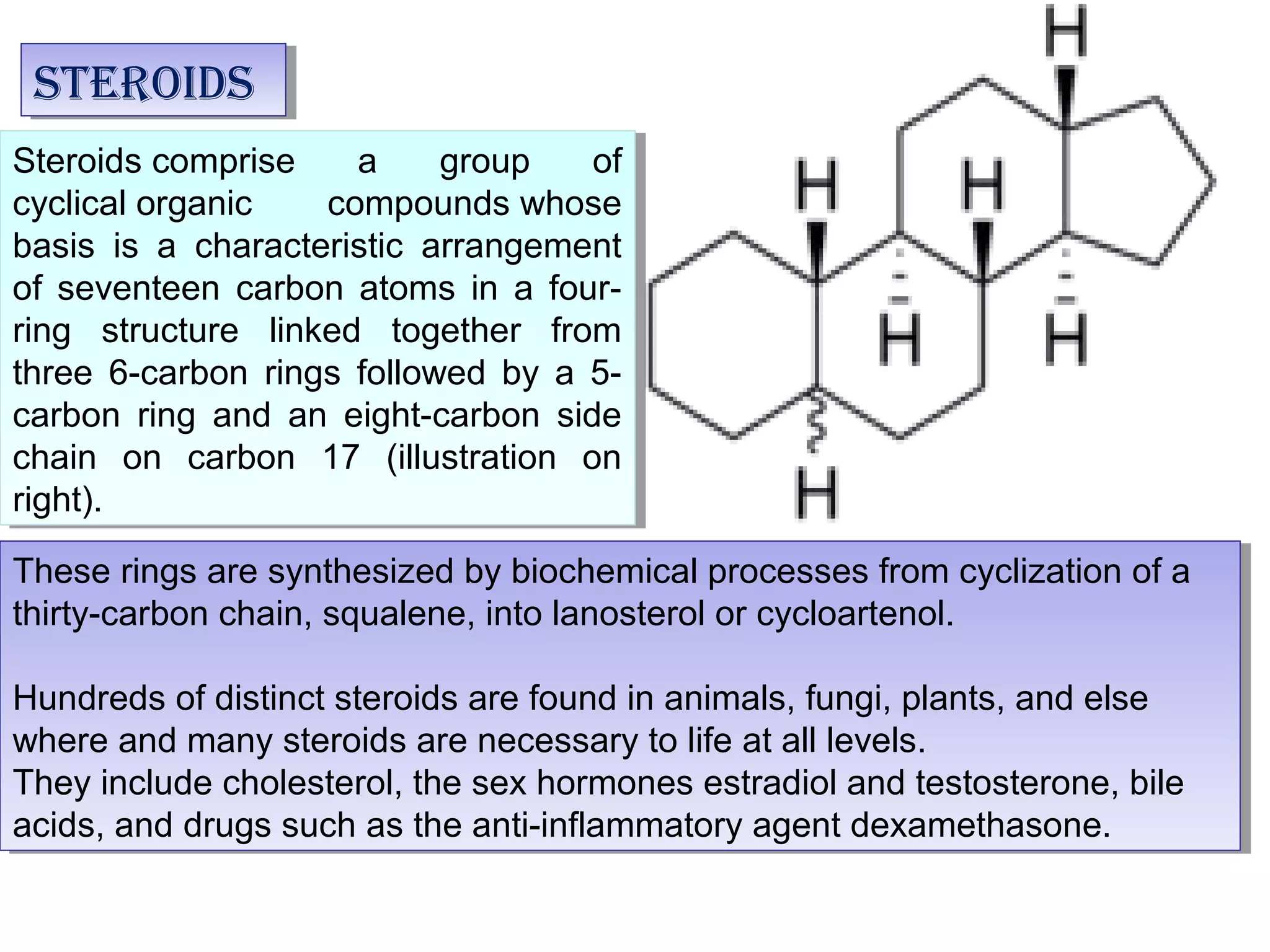
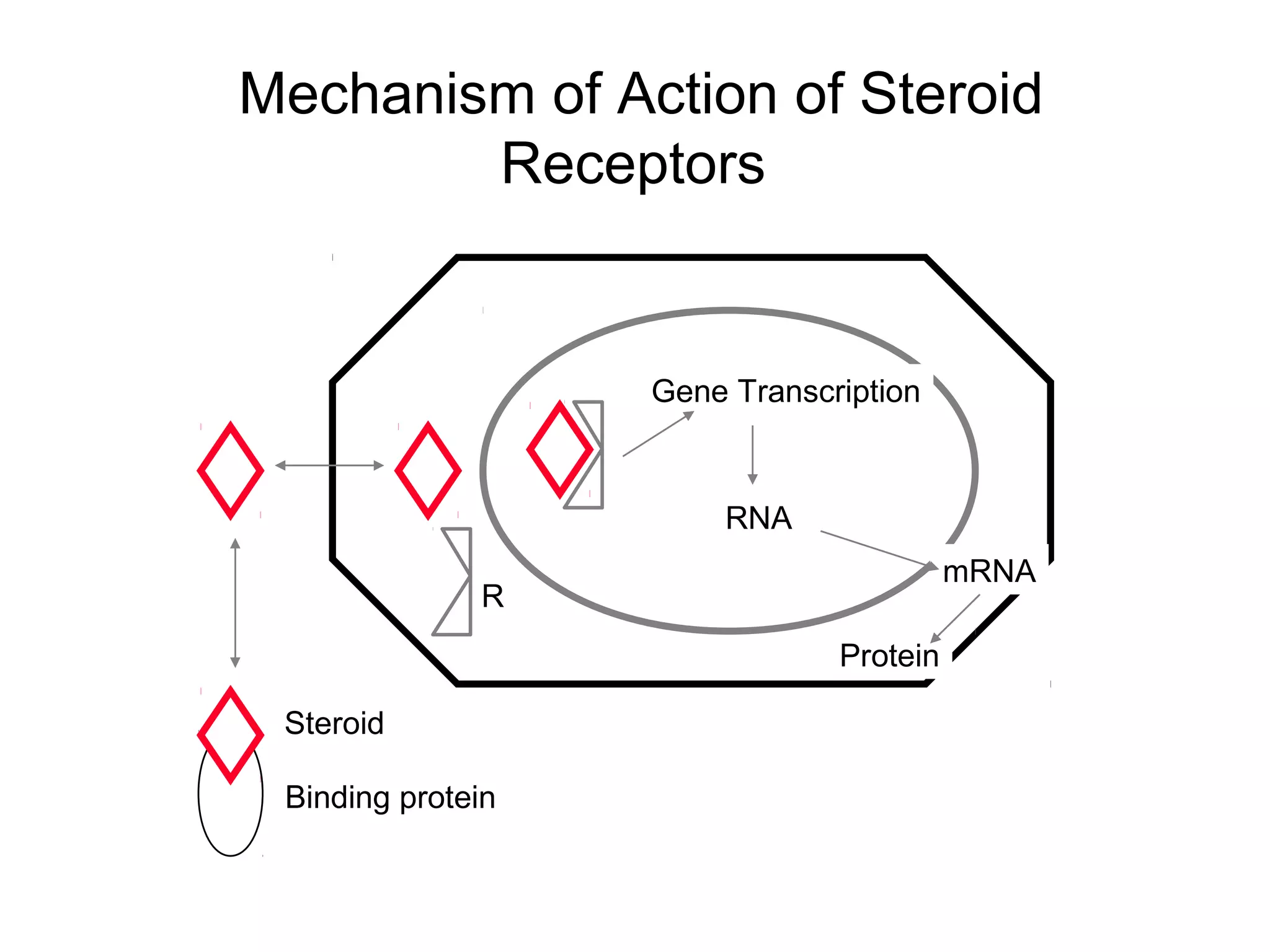
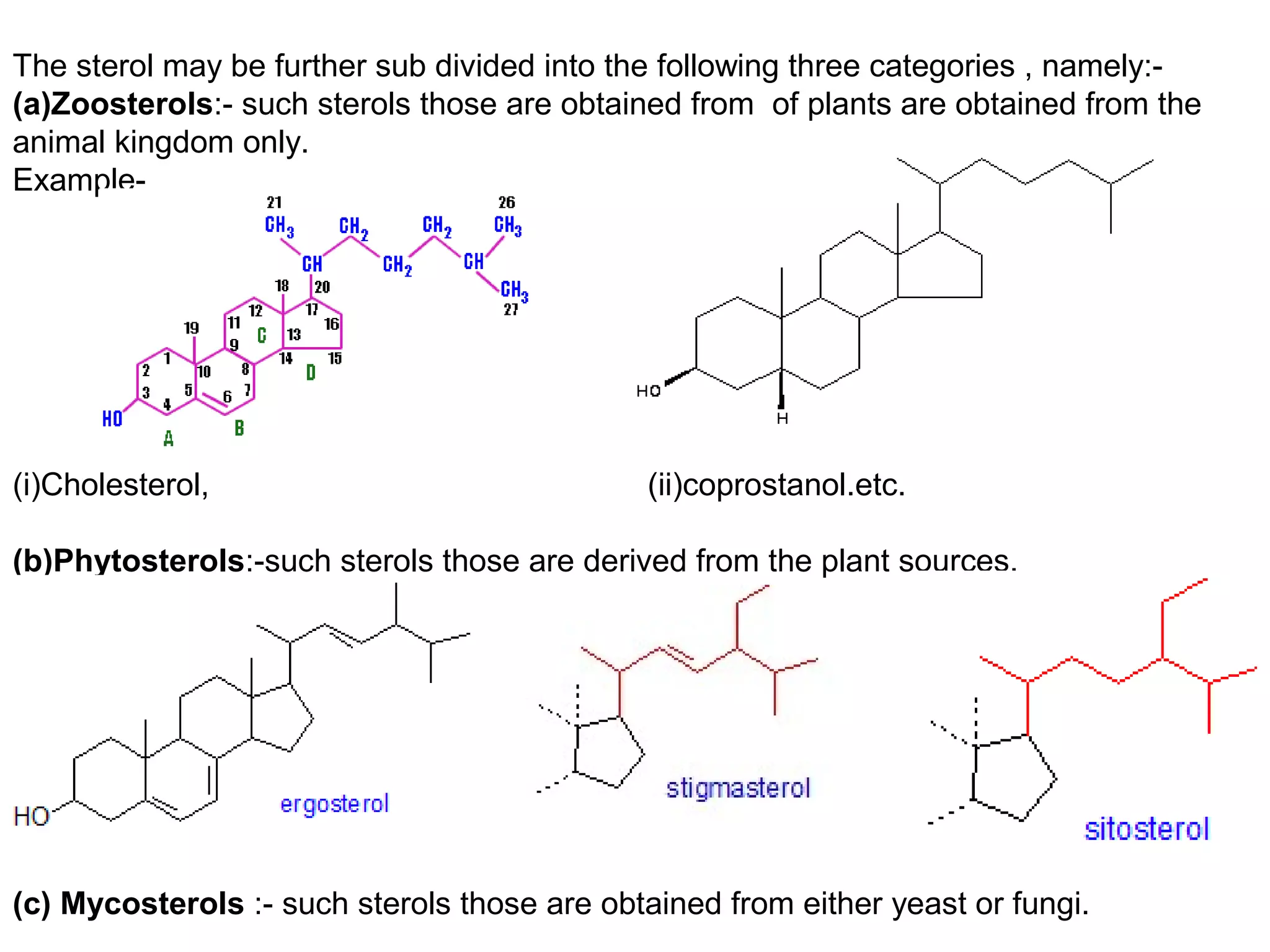
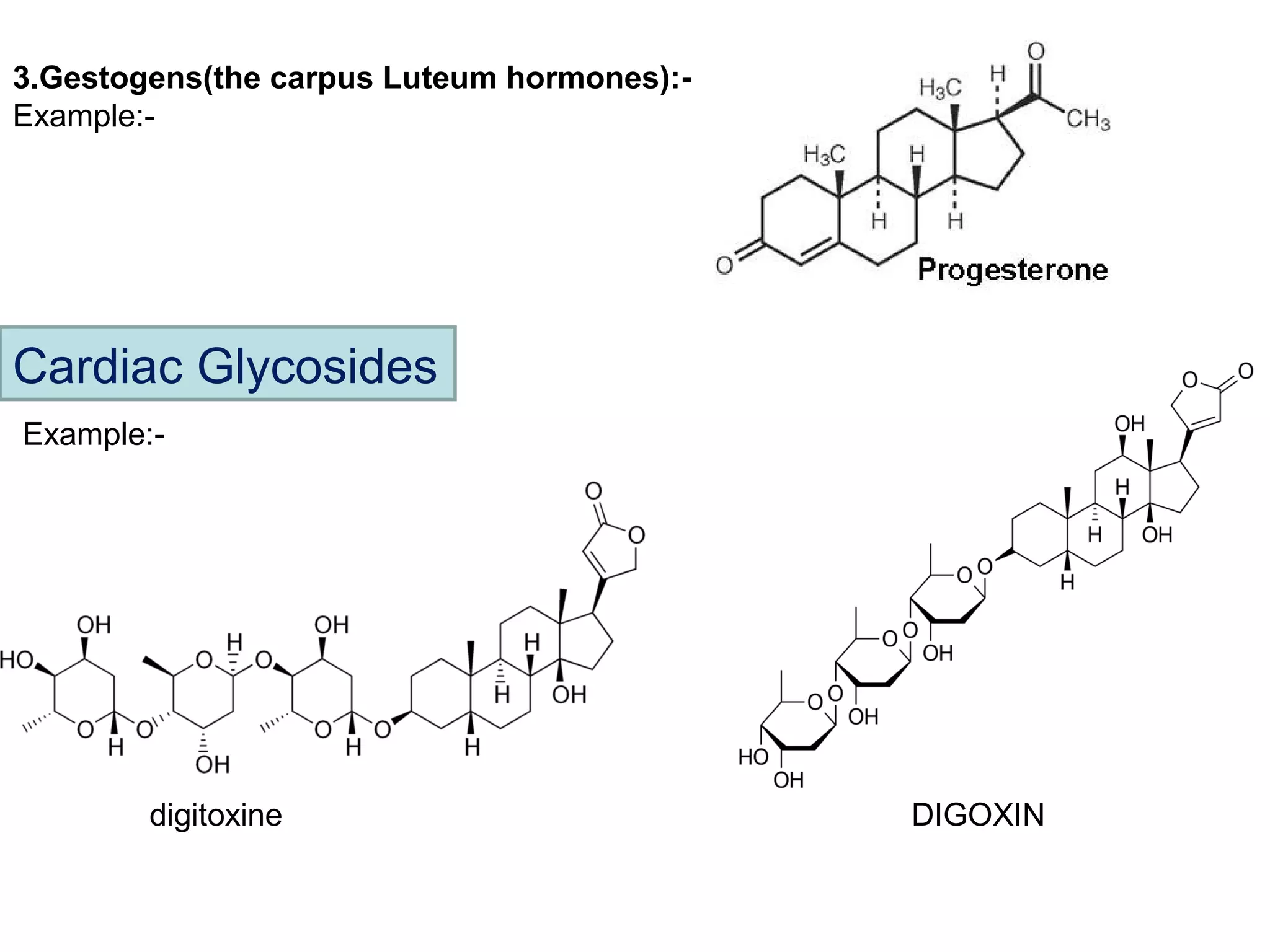
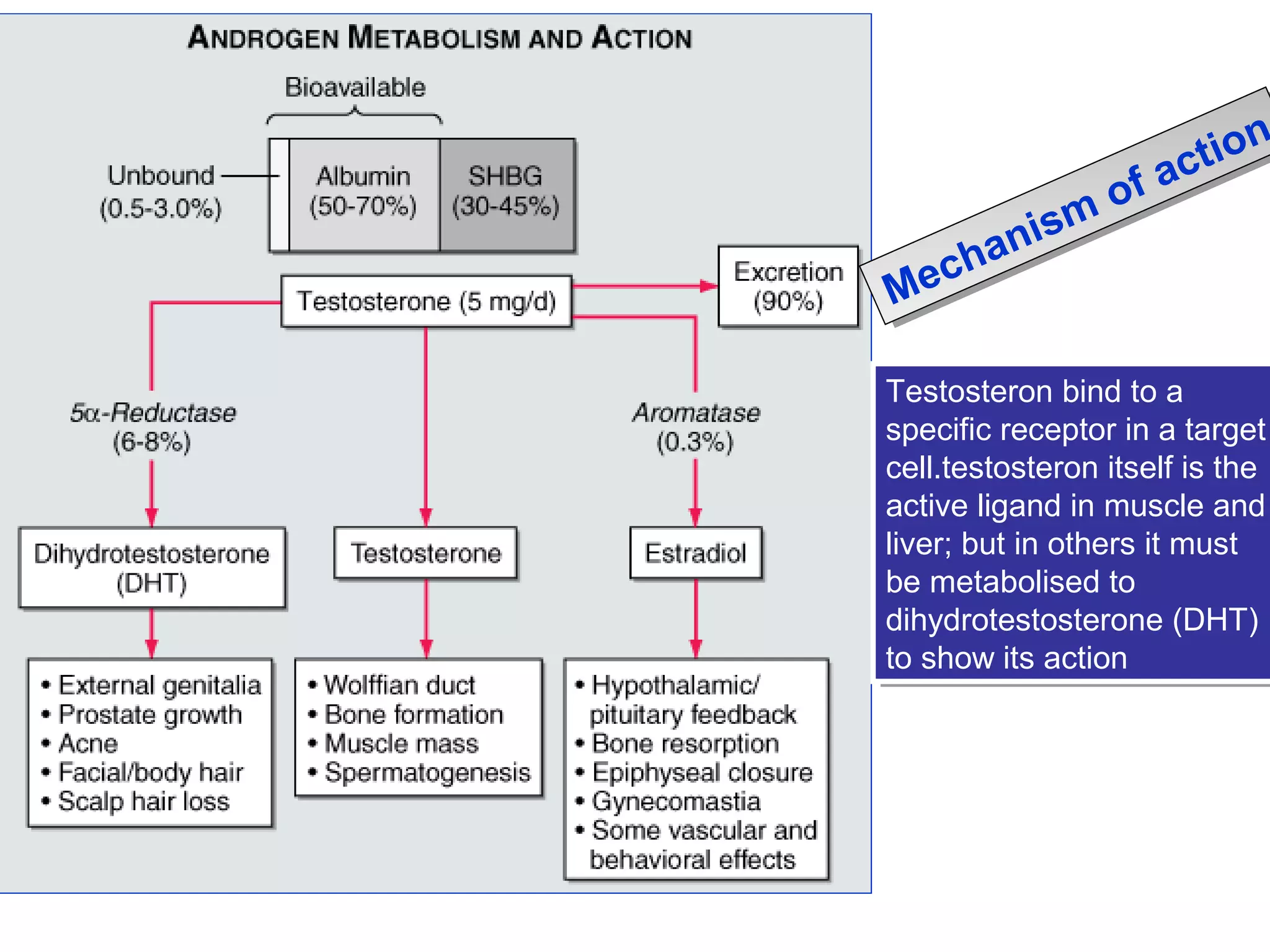
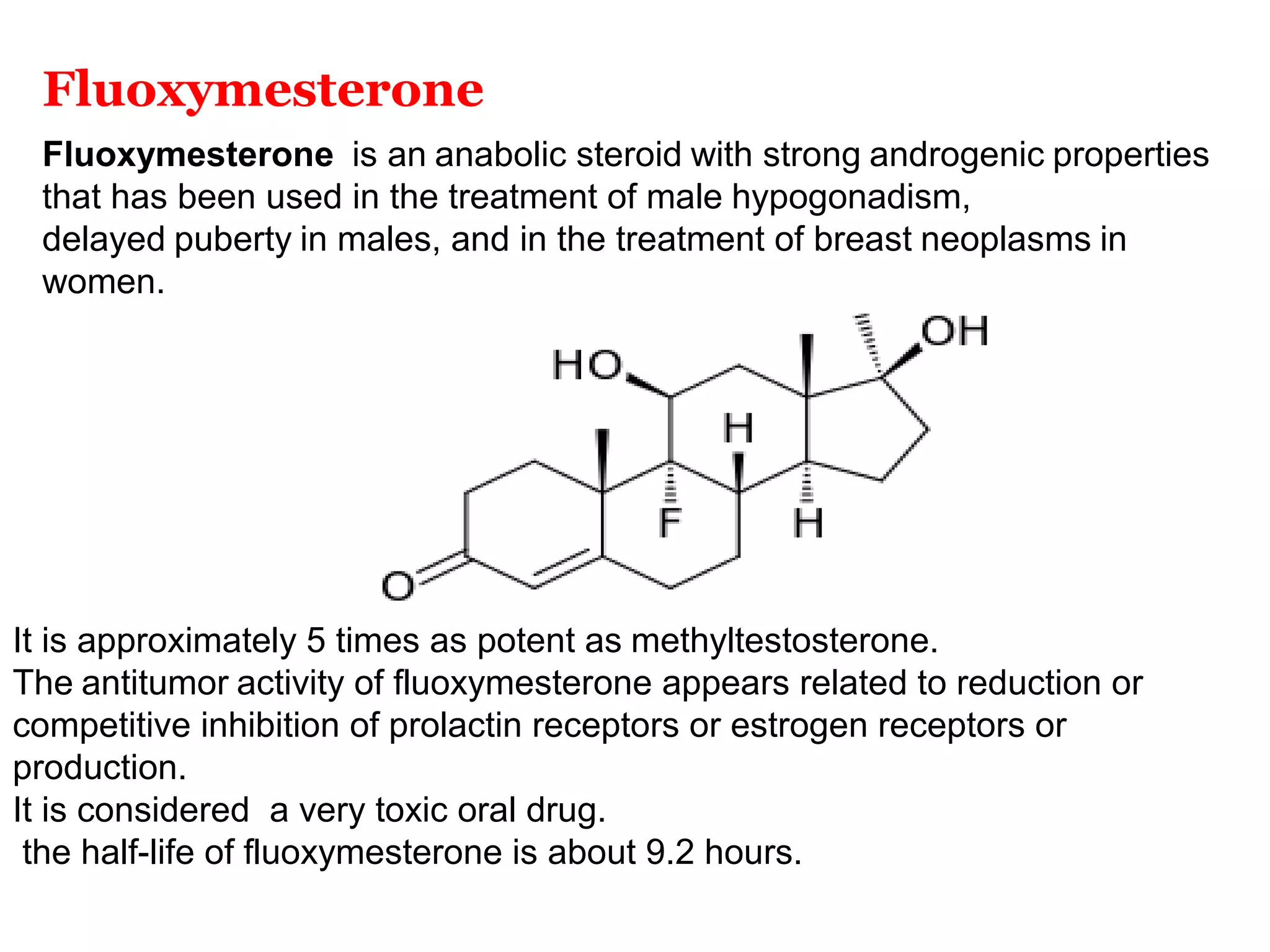
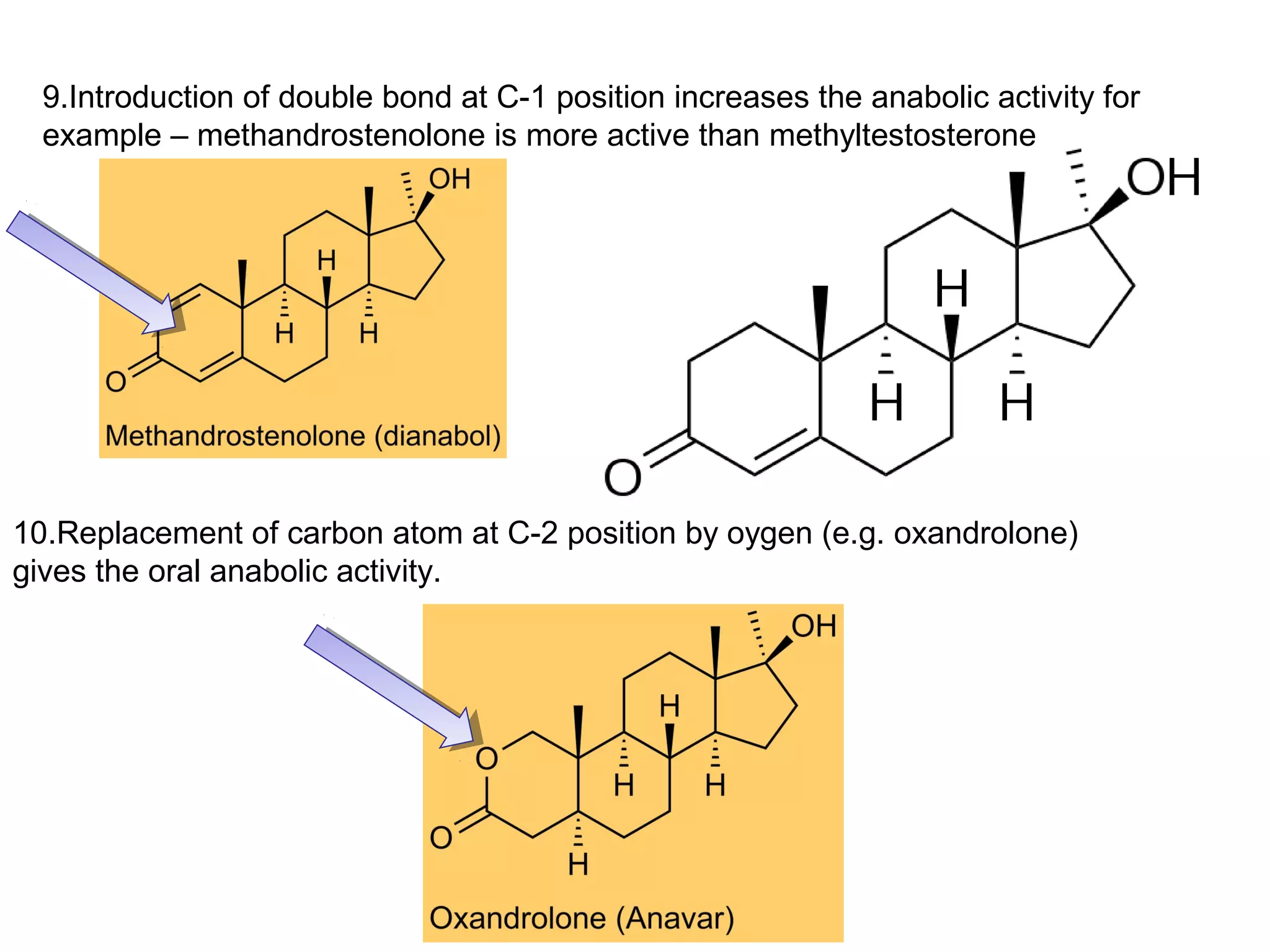
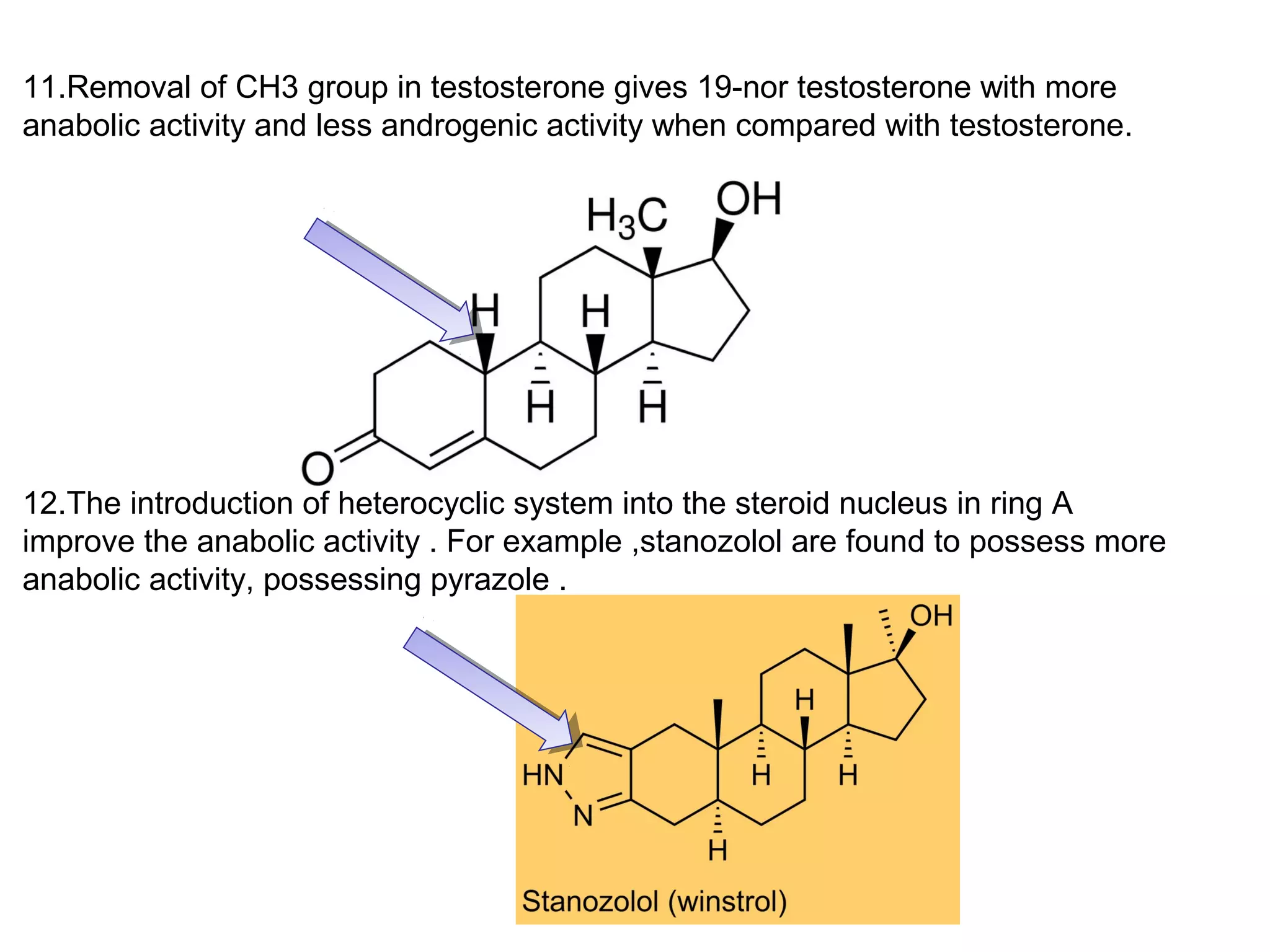
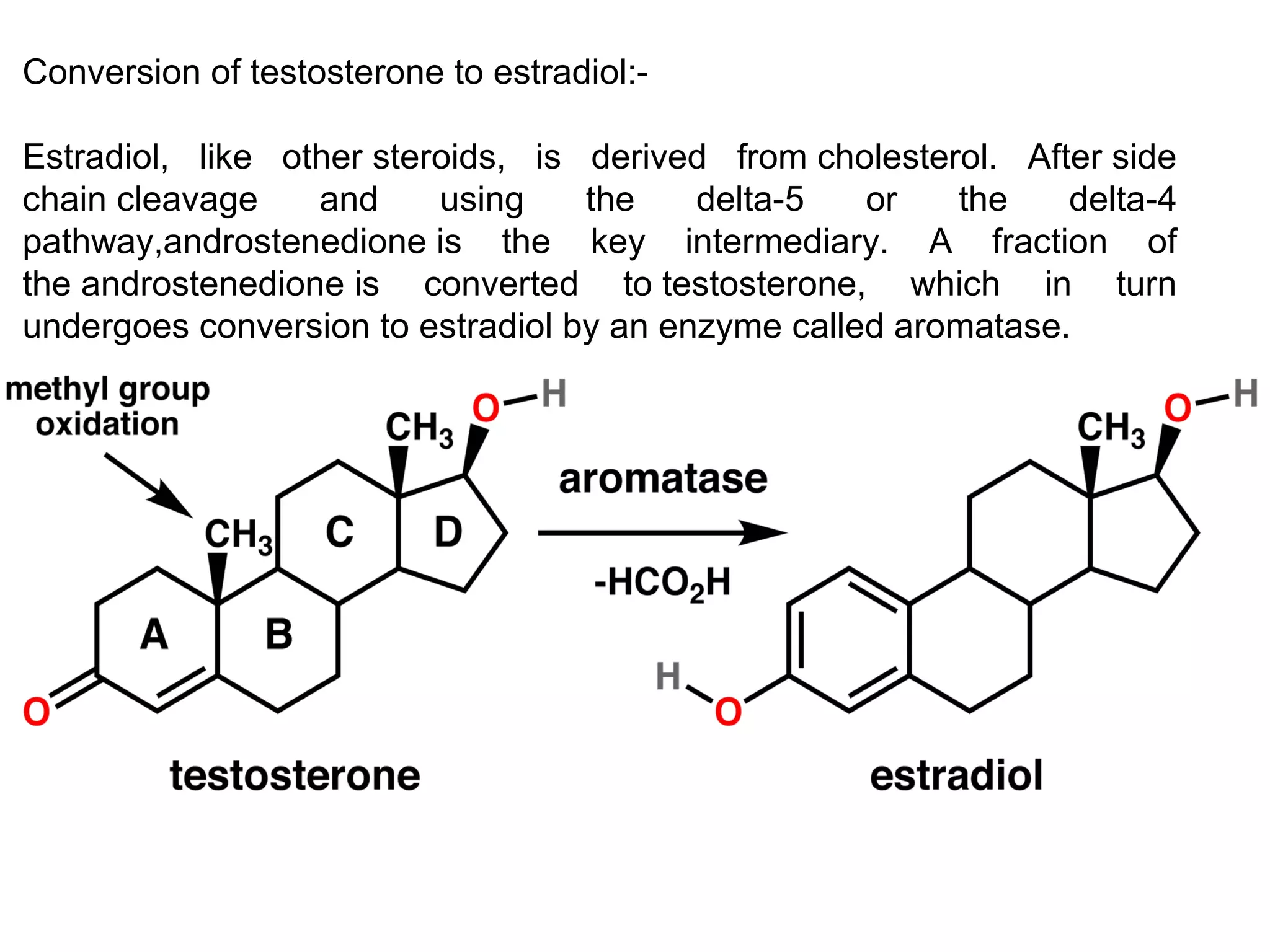
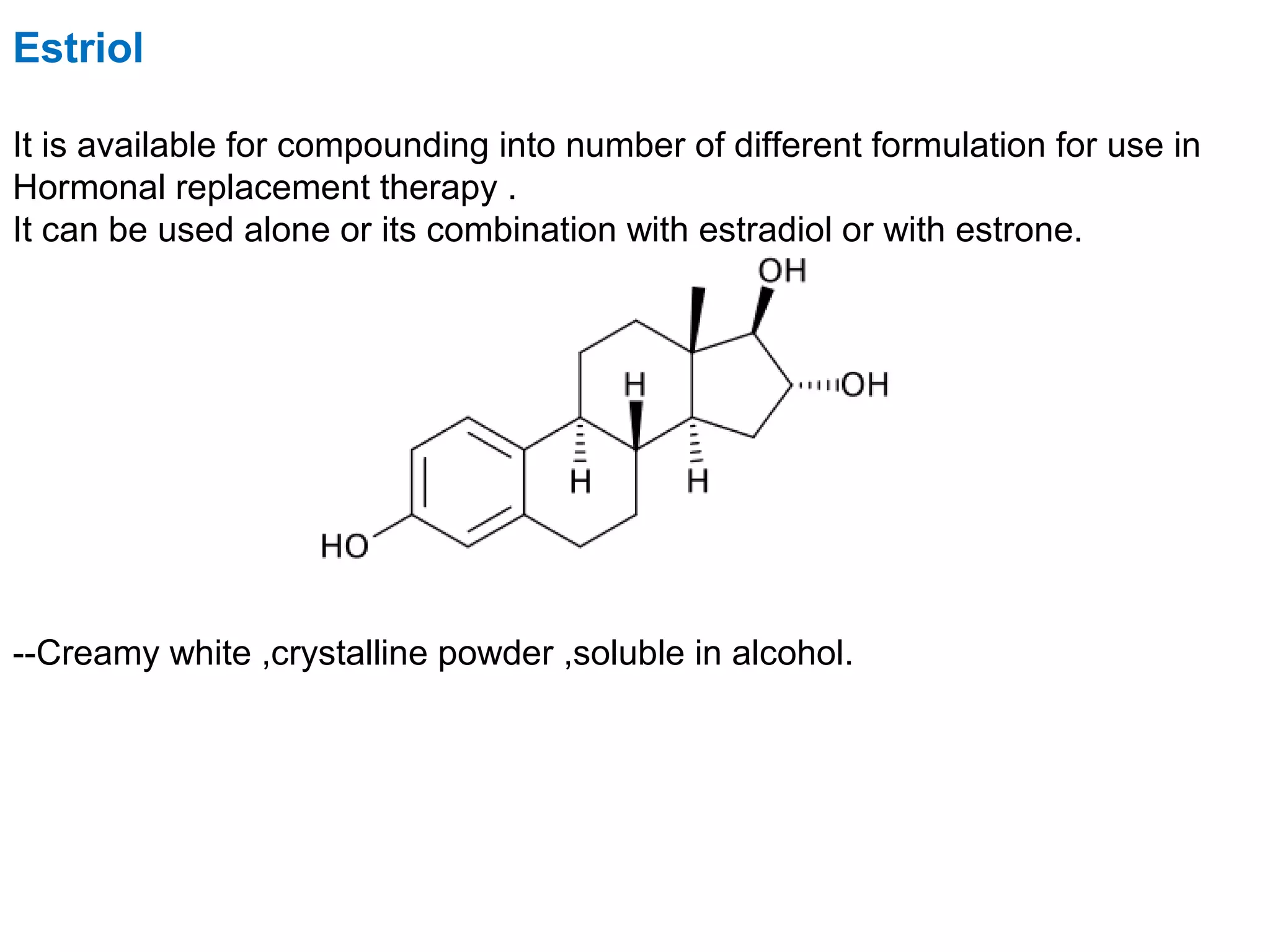
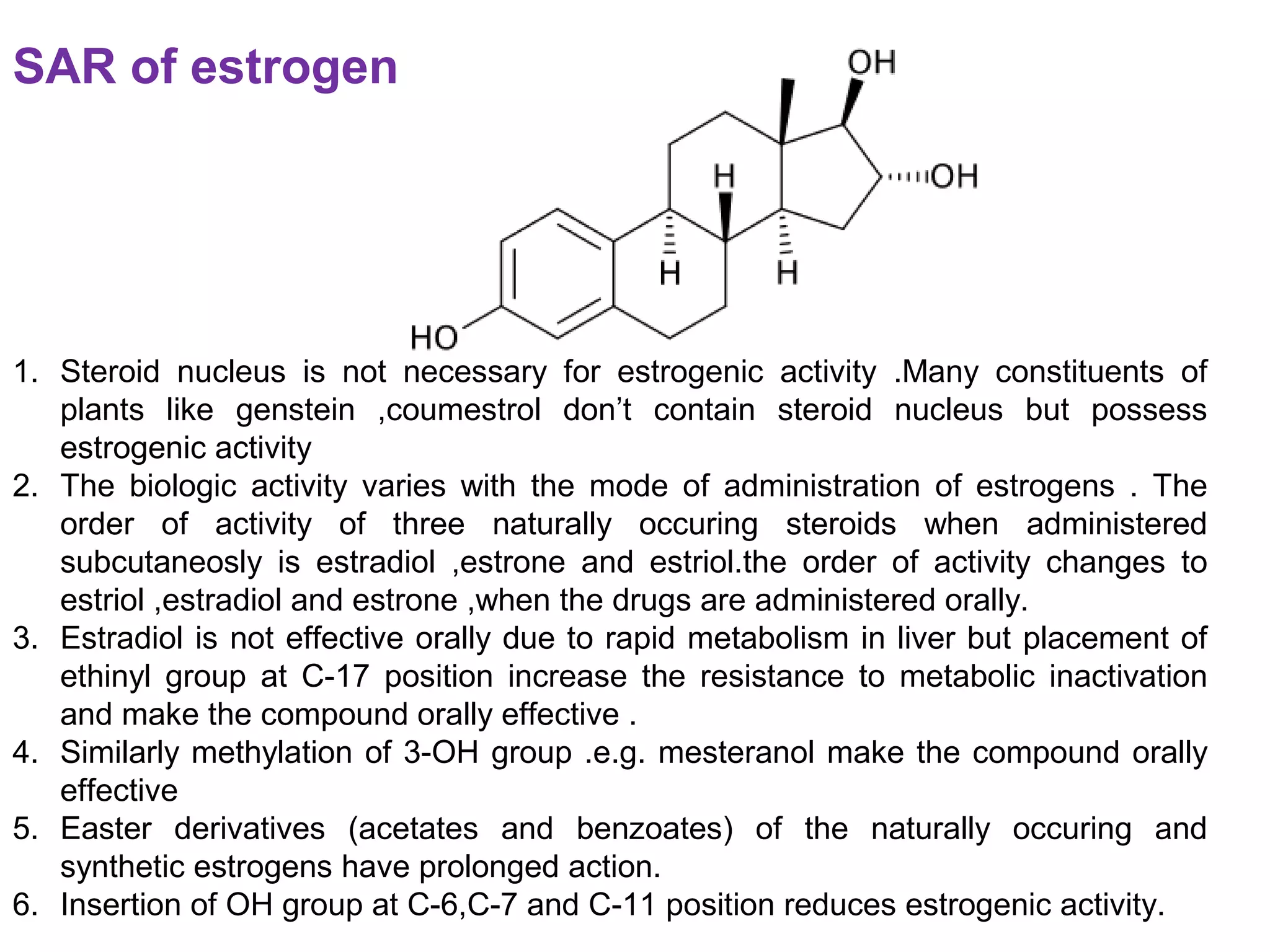
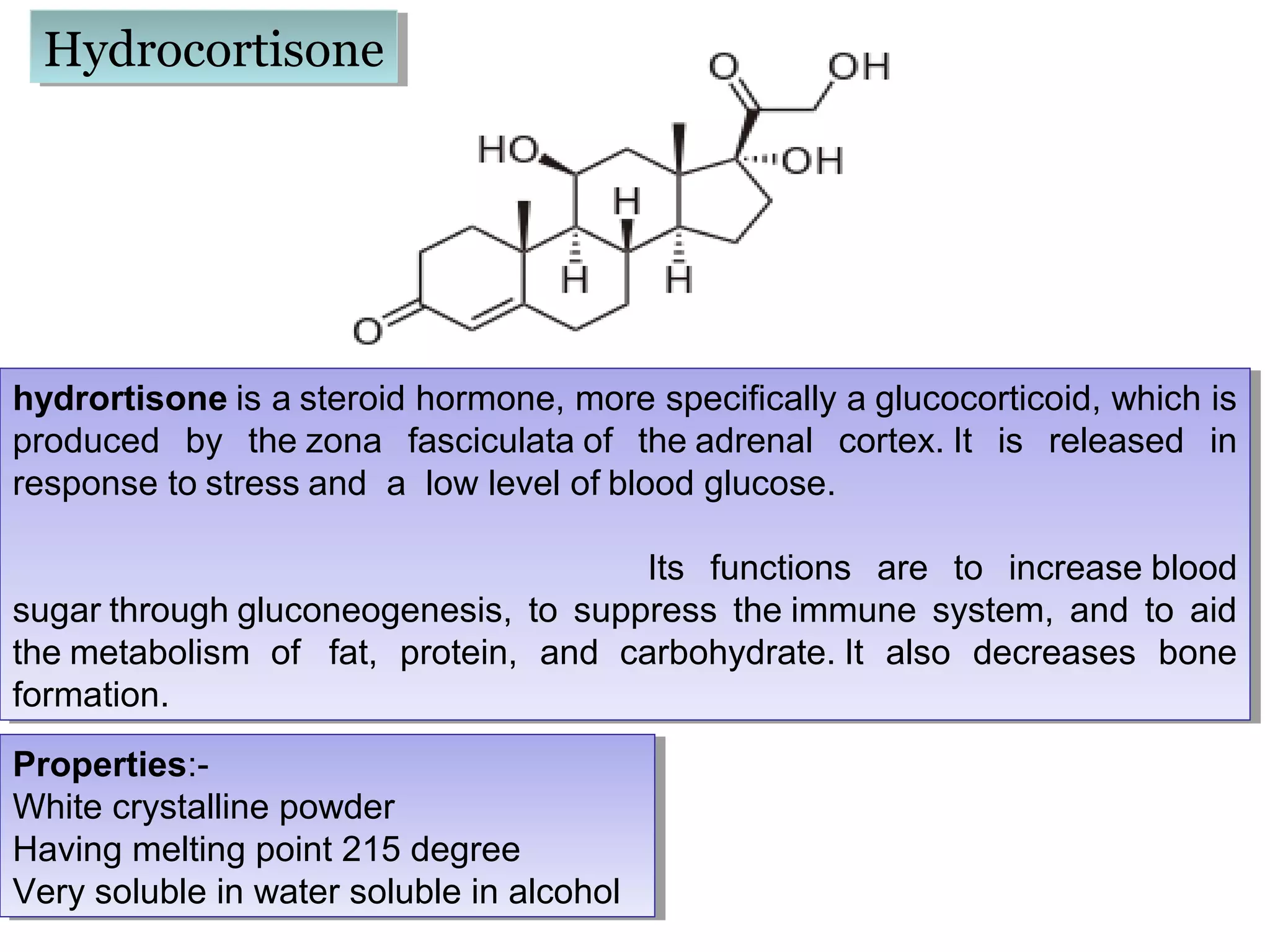
The document discusses steroids, which are cyclical organic compounds composed of 17 carbon atoms arranged in four rings. Steroids include cholesterol, sex hormones like testosterone and estradiol, bile acids, and drugs like dexamethasone. They are classified based on the substituent group at carbon 17 and include classes like sterols, sex hormones, cardiac glycosides, bile acids, and sapogenins. Specific steroids discussed in more detail include testosterone, estradiol, progesterone, and various androgens and glucocorticoids.