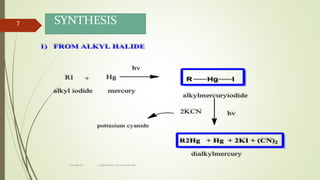
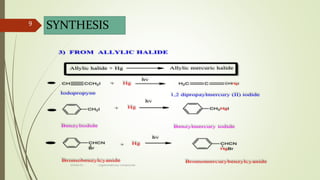
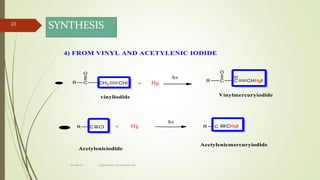
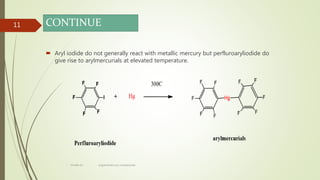
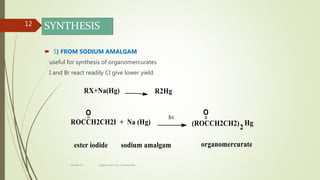
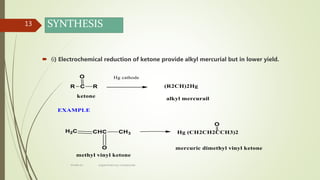
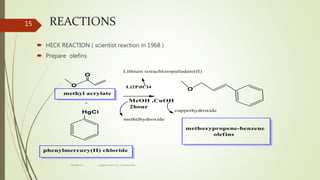
This document presents information on organomercury compounds. It introduces organomercury compounds and notes that the Hg-C bond is typically stable but sensitive to light. It discusses the structure, preparation, reactions and examples of important organomercury compounds like methylmercury and dimethyl mercury. The preparation methods covered include the direct reaction of hydrocarbons with mercury salts and the use of sodium amalgam. Key reactions discussed are the Heck reaction and oxymercuration-demercuration reactions. Applications mentioned include use as fungicides, catalysts, and in medicines.











![Reaction
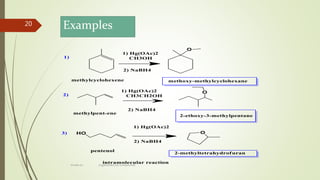
OXYMERCURATION OF AKENES
When an alkene is treated with an alcohol in the presence of mercuric acetate
[Hg(OAc)2] it will add to the more substituted position of the alkene (Markovnikoff) to
give an ether. The mercury can then be removed using NaBH4.
19
rimsha b.r organomercury compounds](https://image.slidesharecdn.com/organomercuarycompounds-210502102849/85/Organo-mercuary-compounds-19-320.jpg)