This document discusses various methods for determining the structure of terpenoid compounds, including:
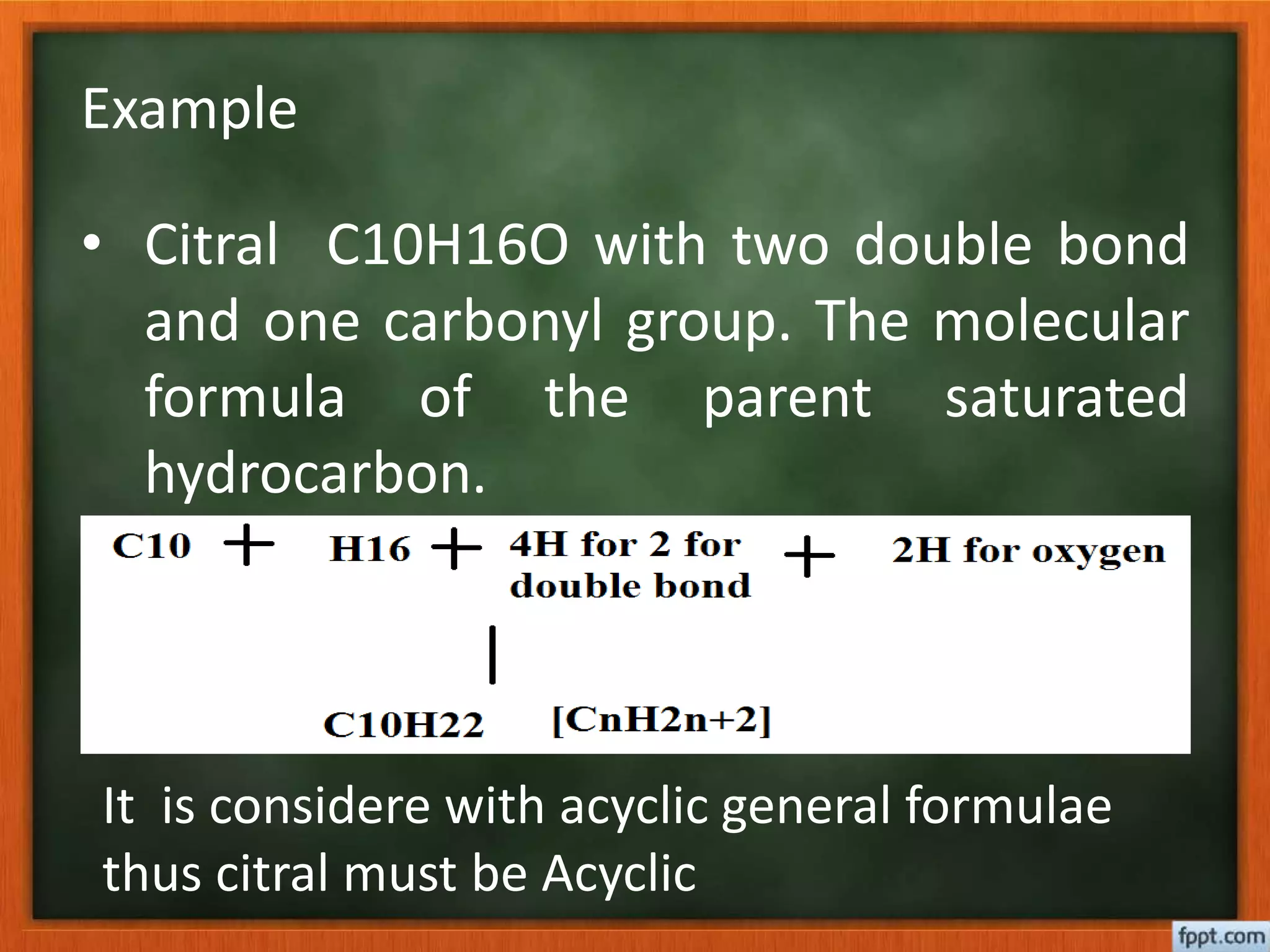
- Elemental analysis and specific rotation to determine molecular formula.
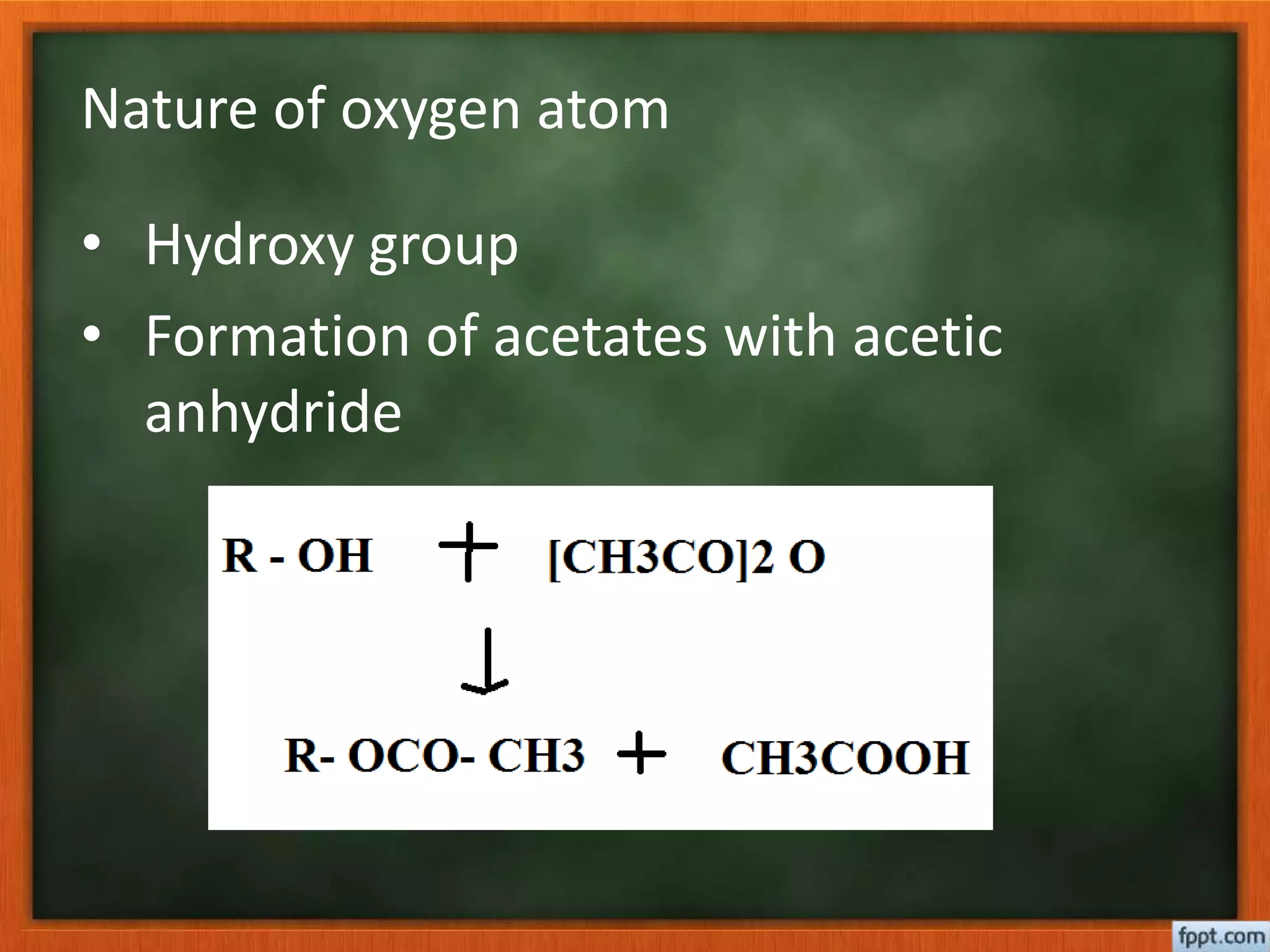
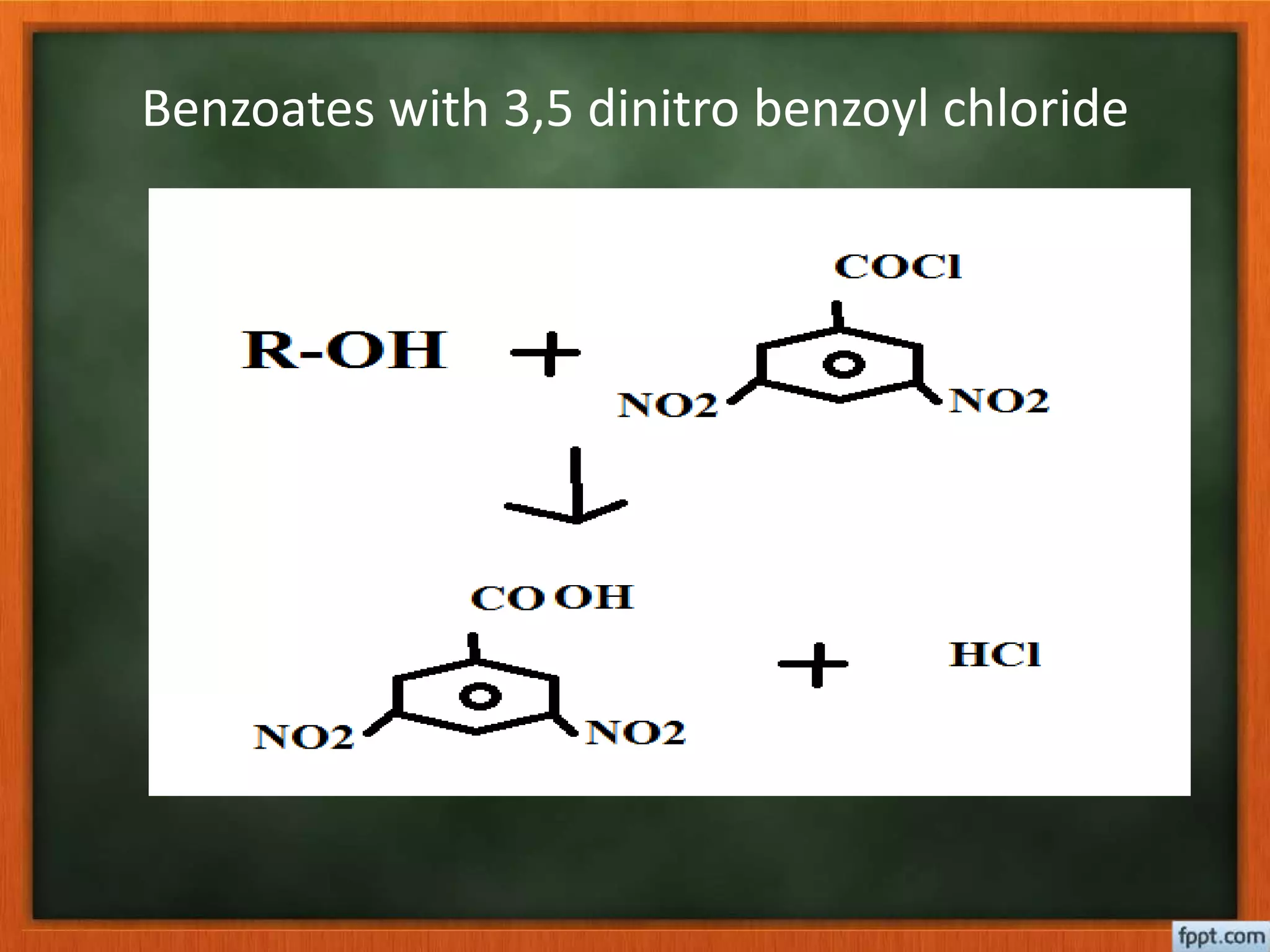
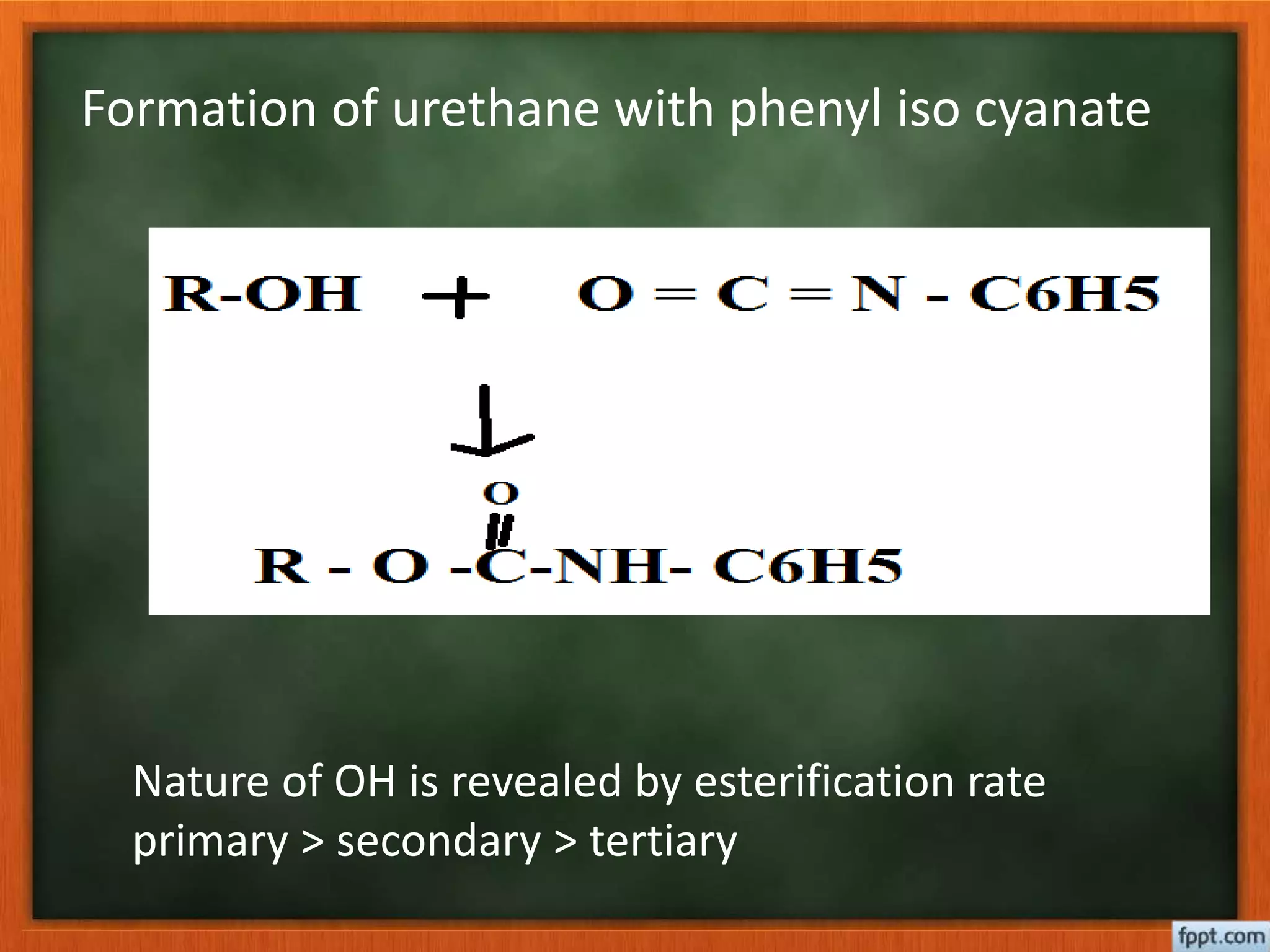
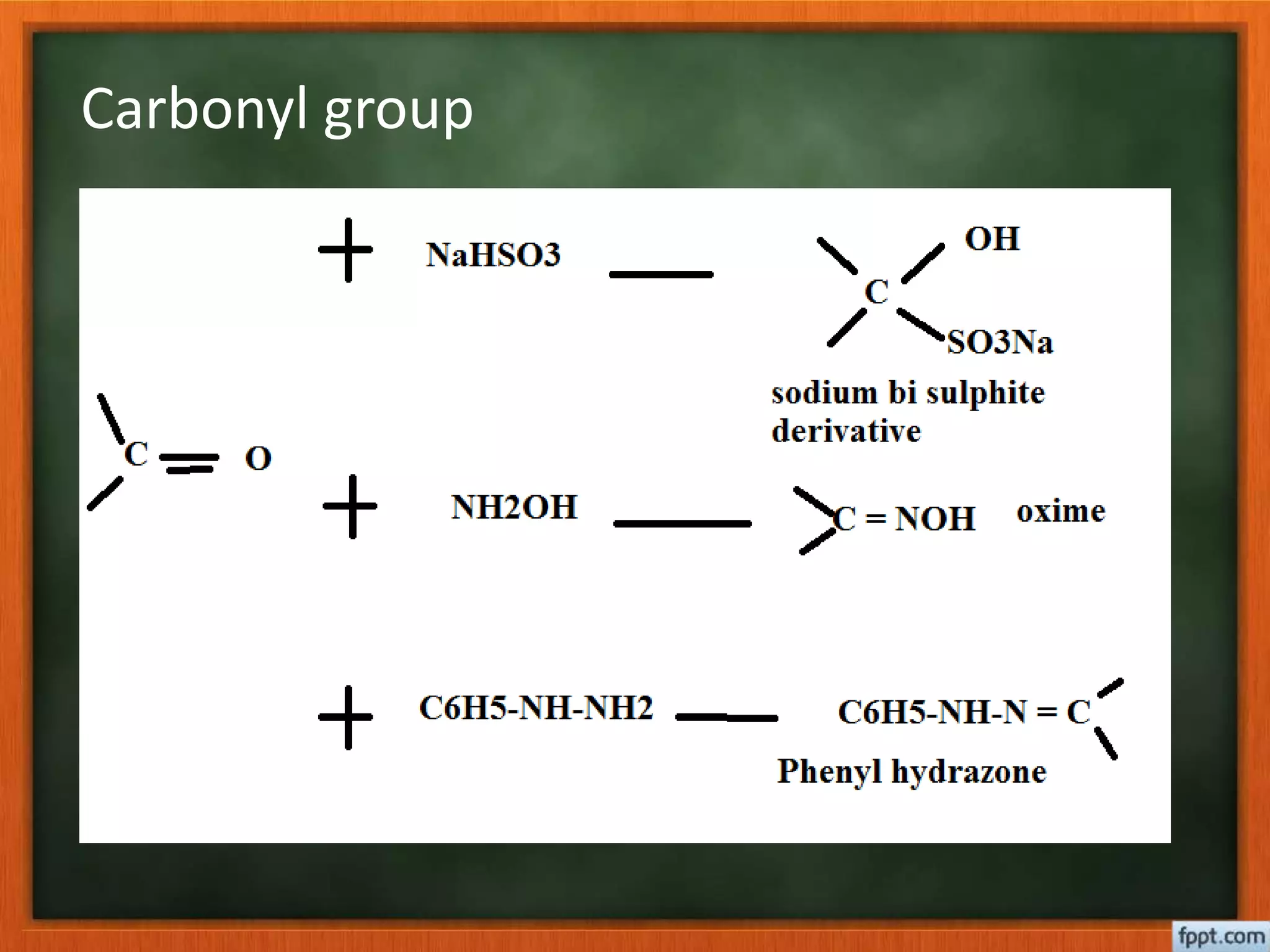
- Esterification rates to determine location of functional groups like hydroxyl and carboxyl.
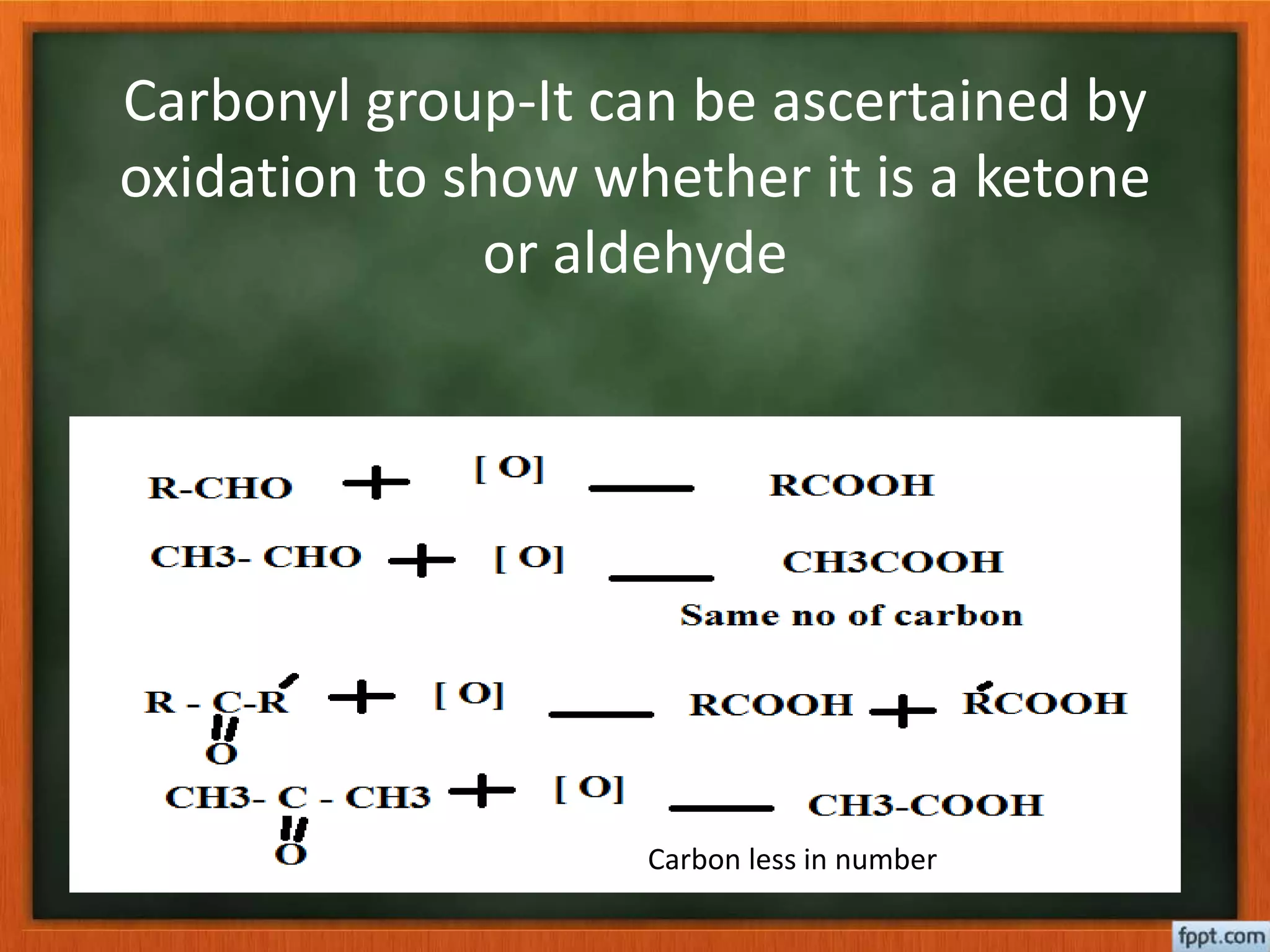
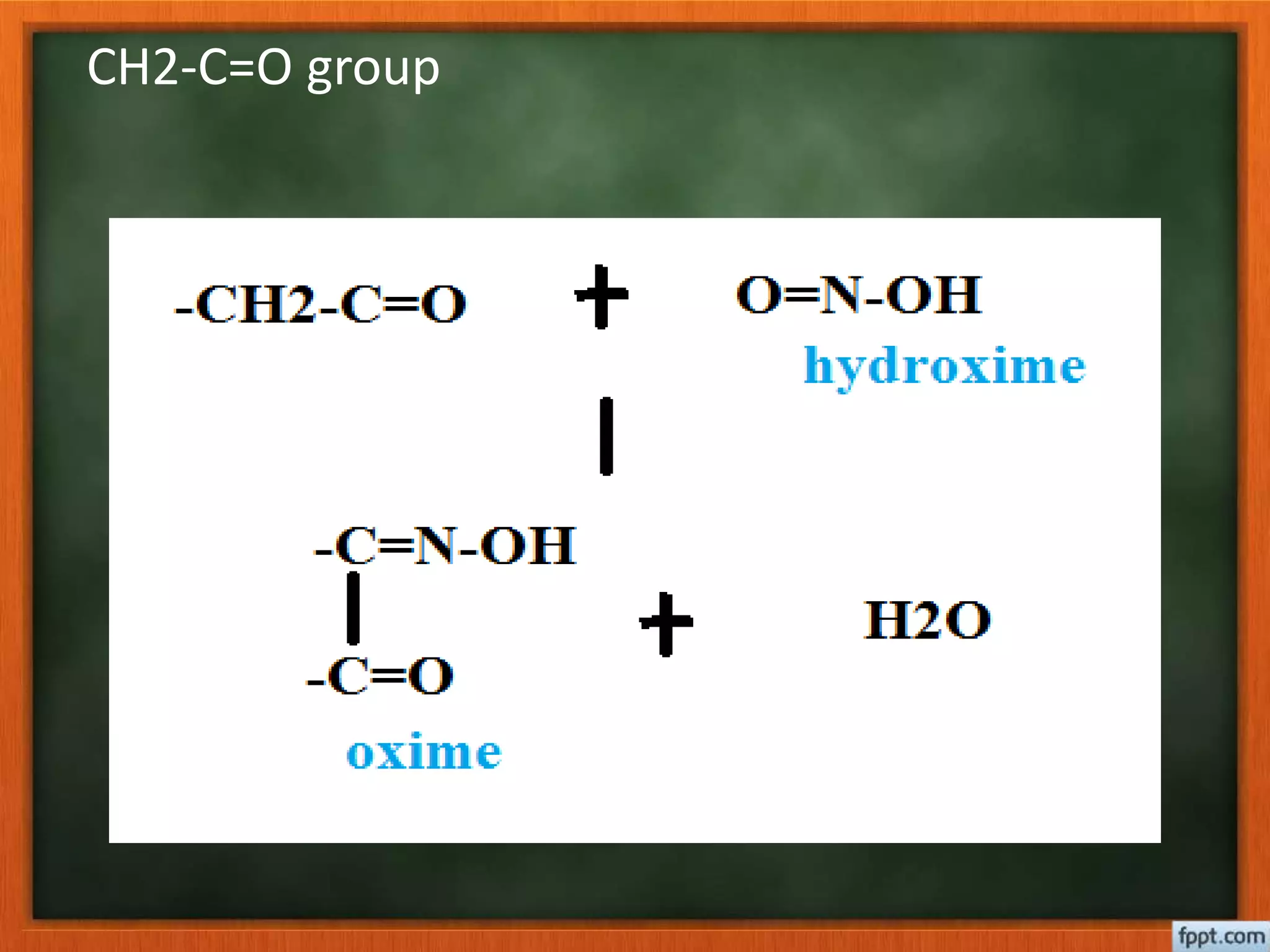
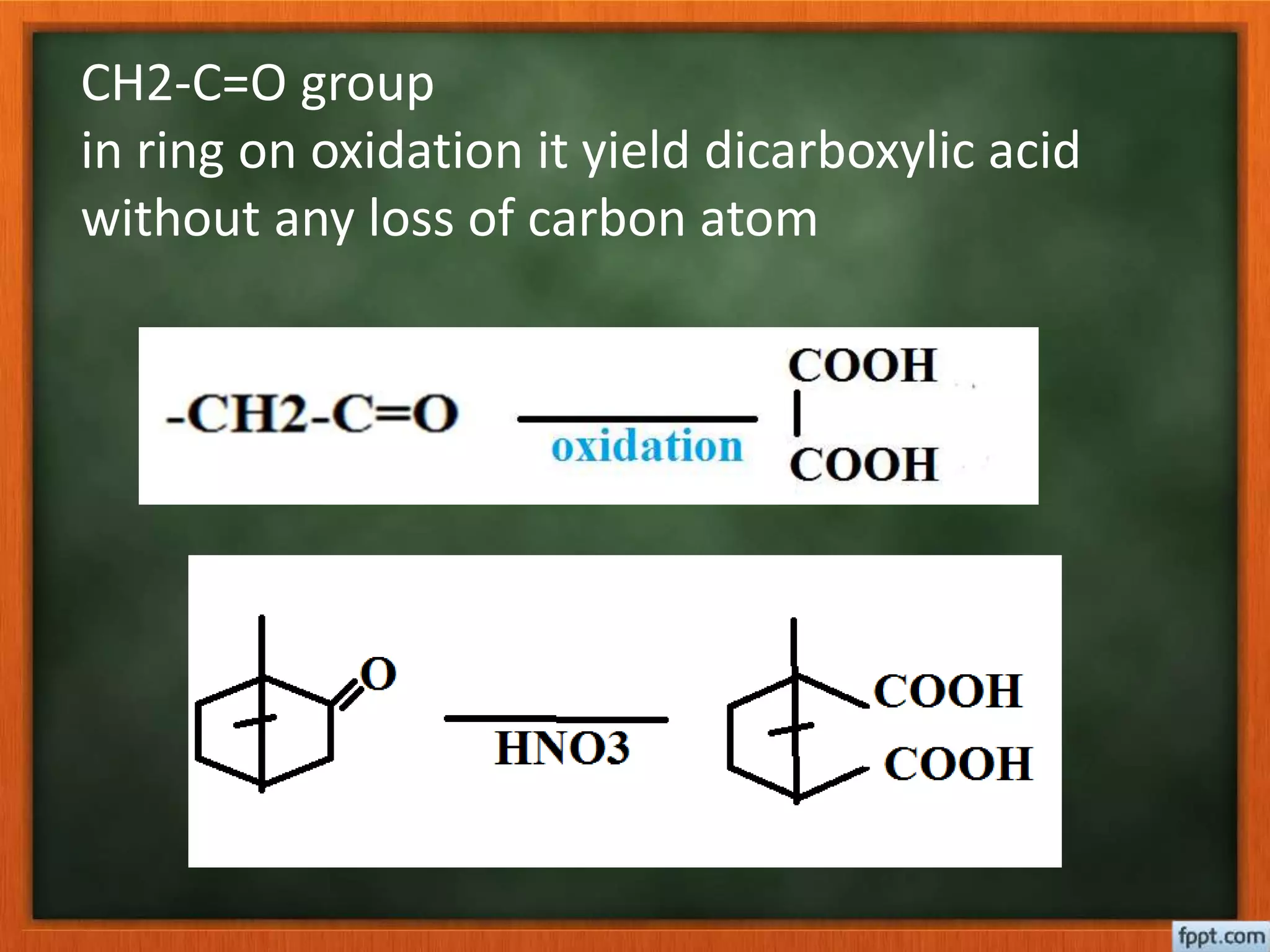
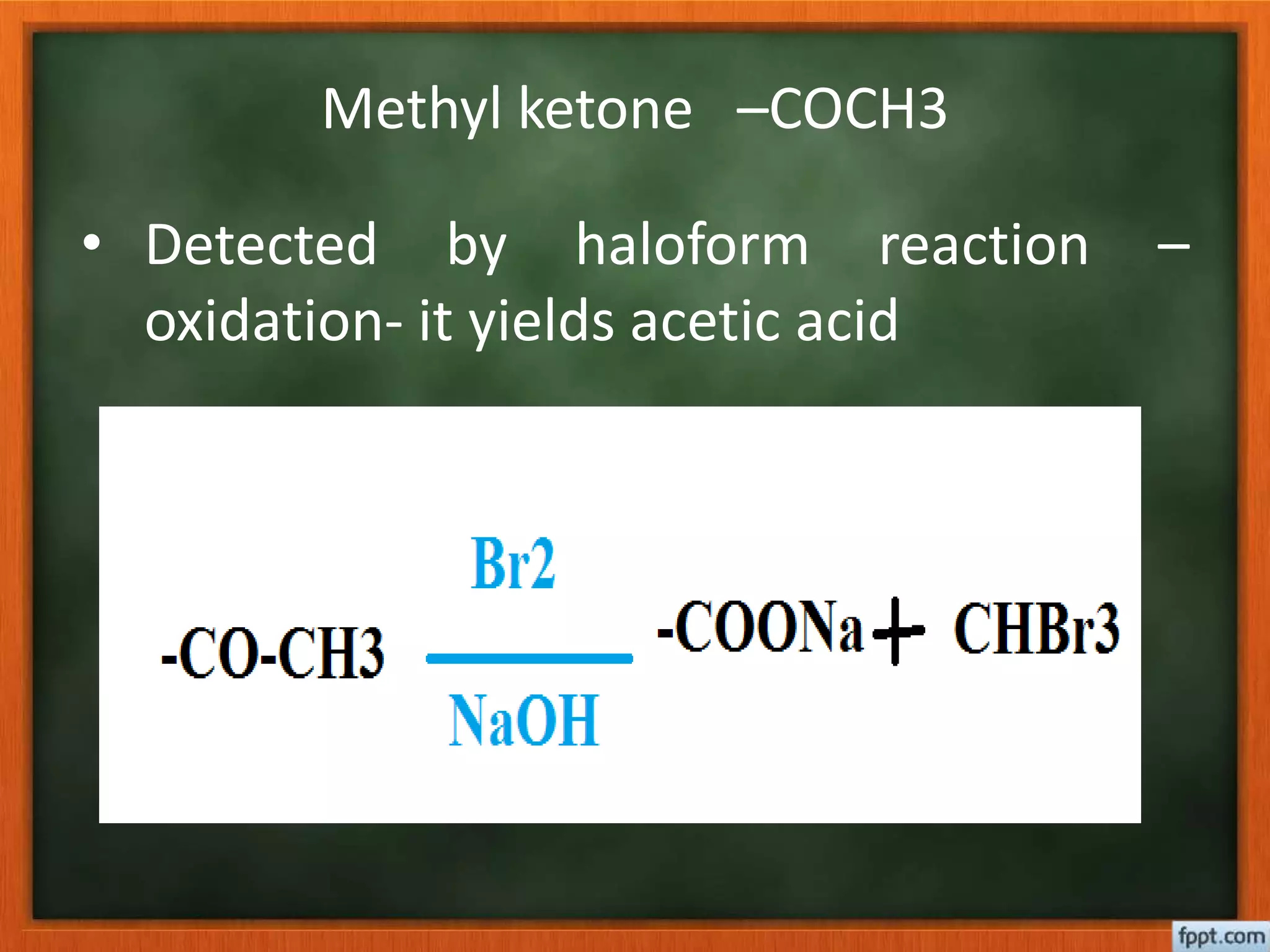
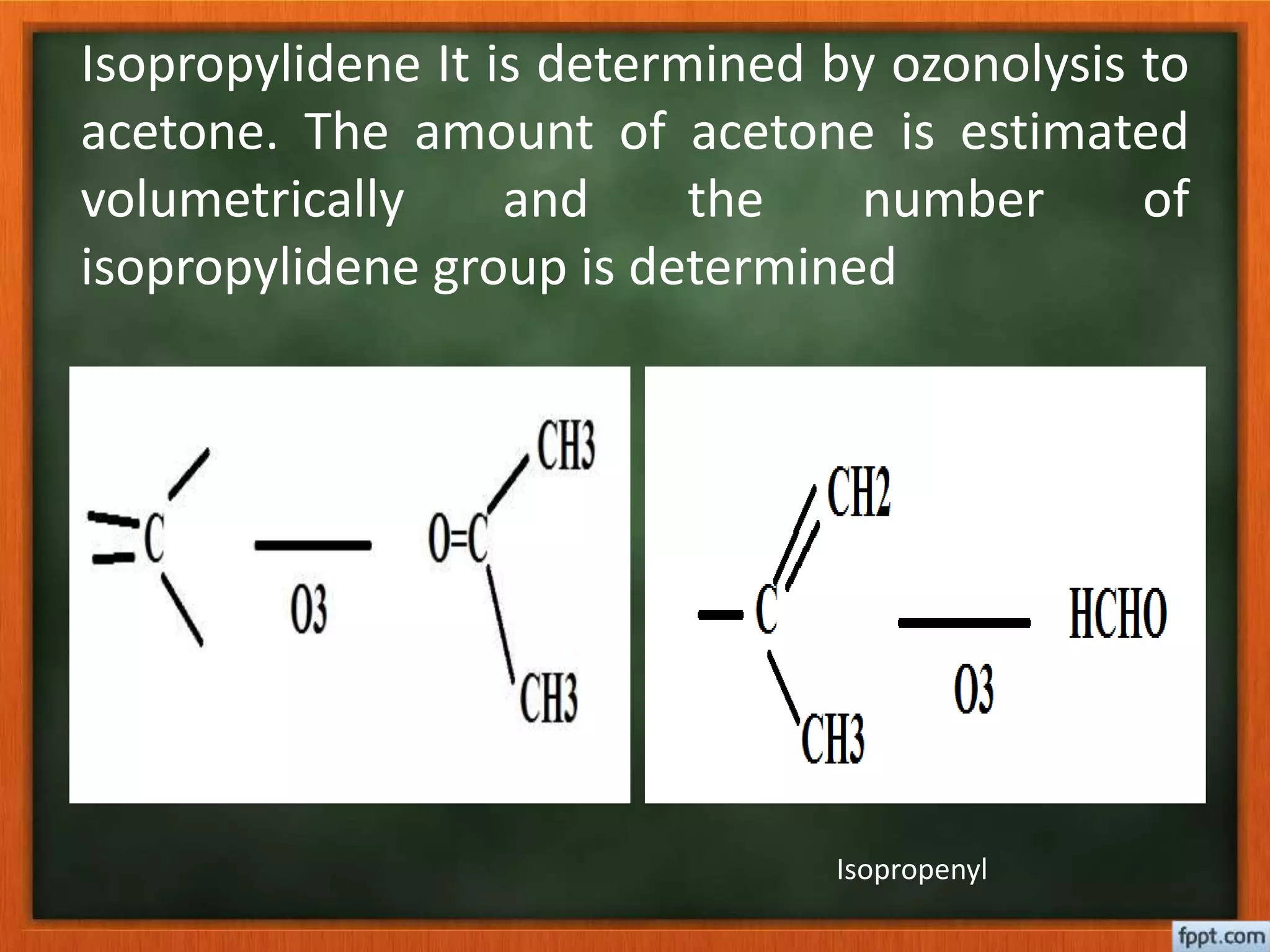
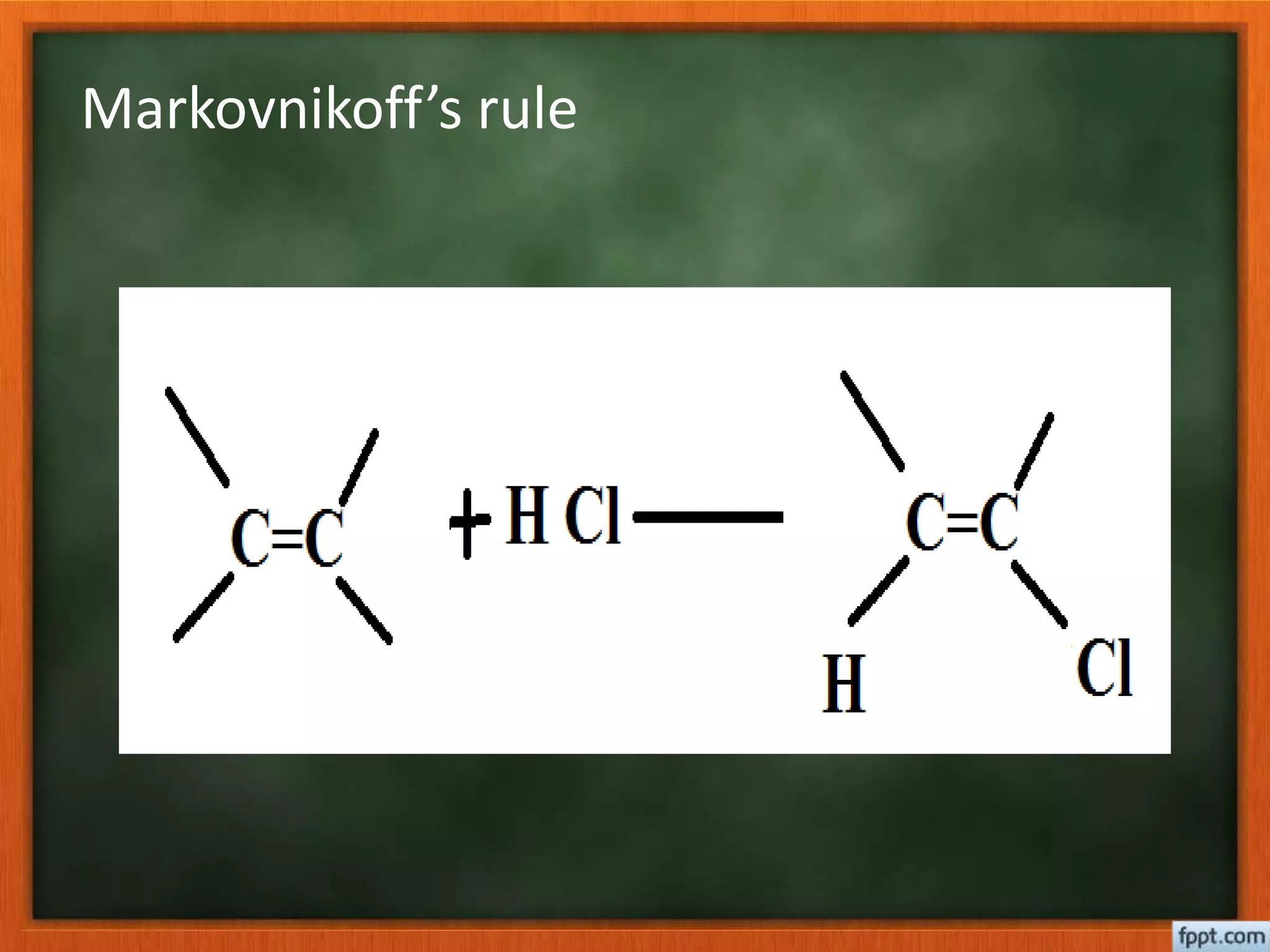
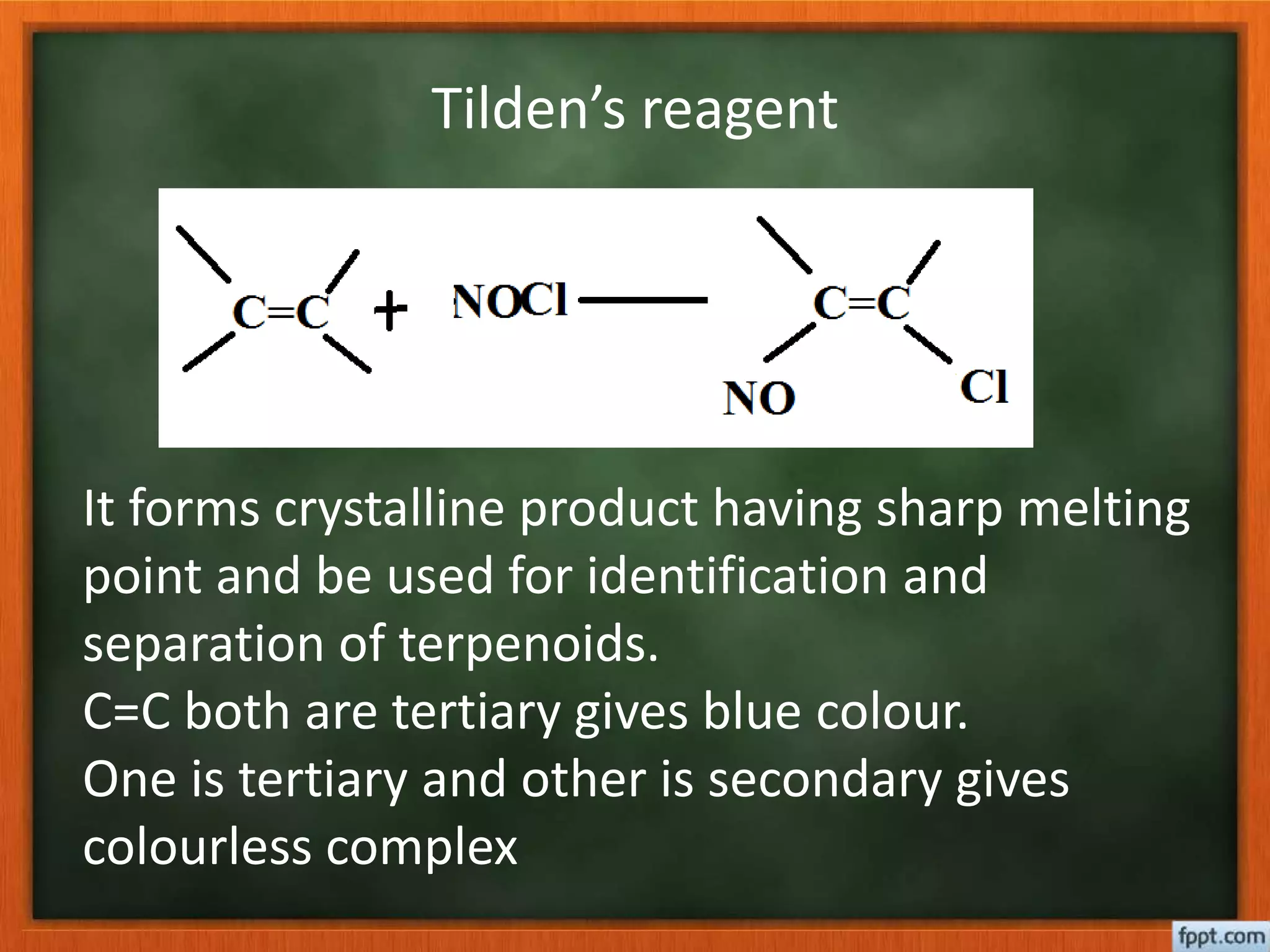
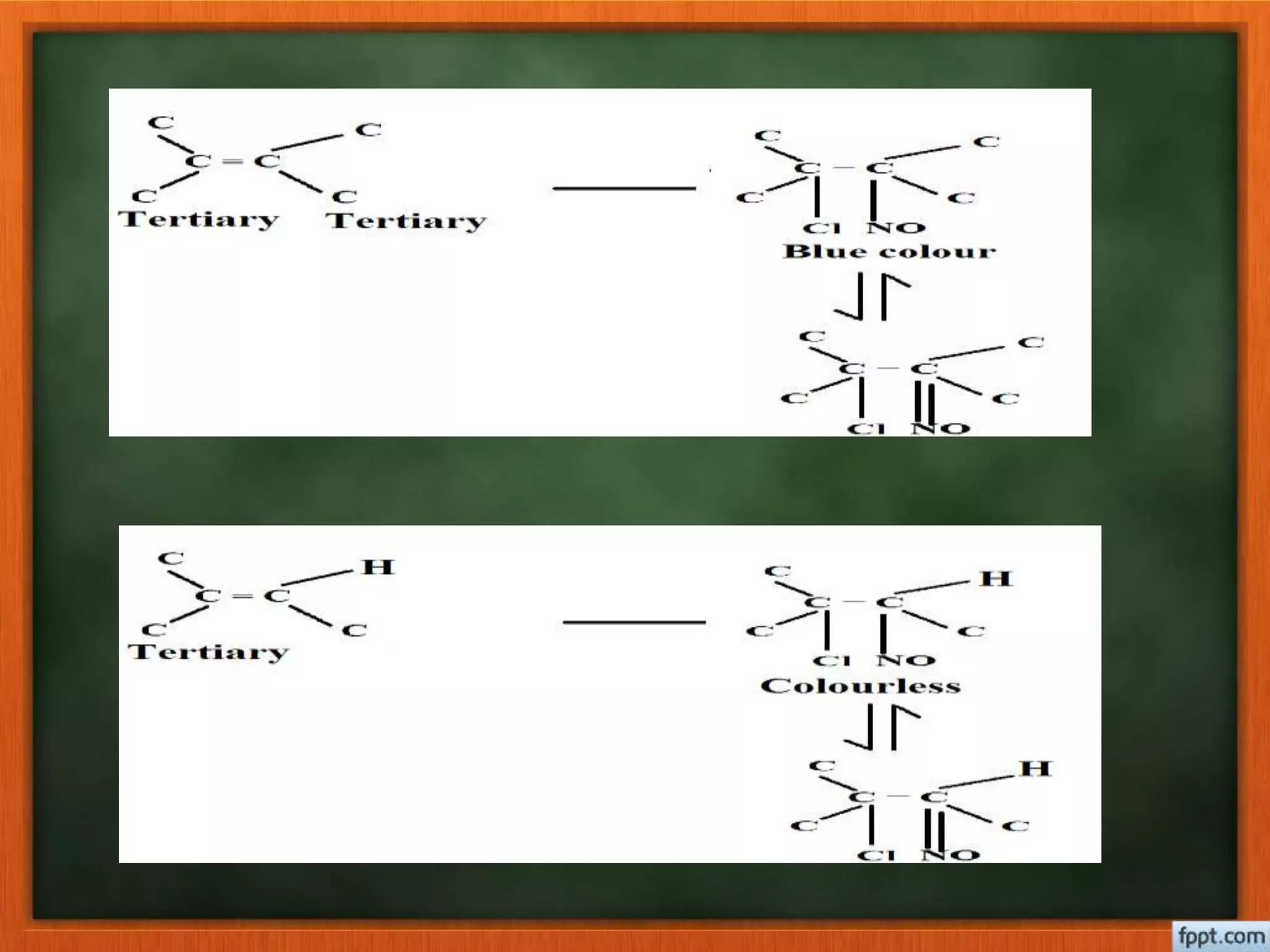
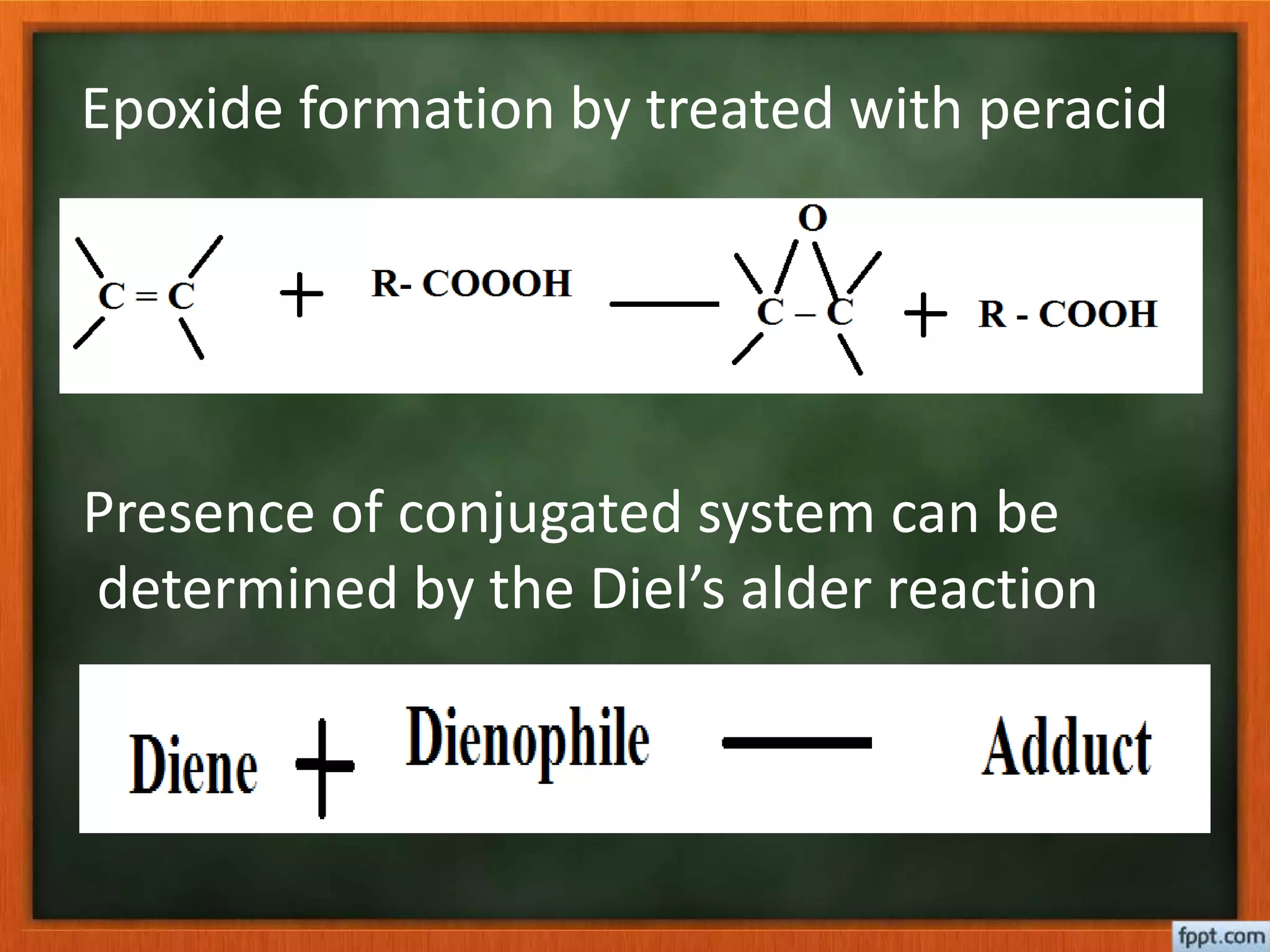
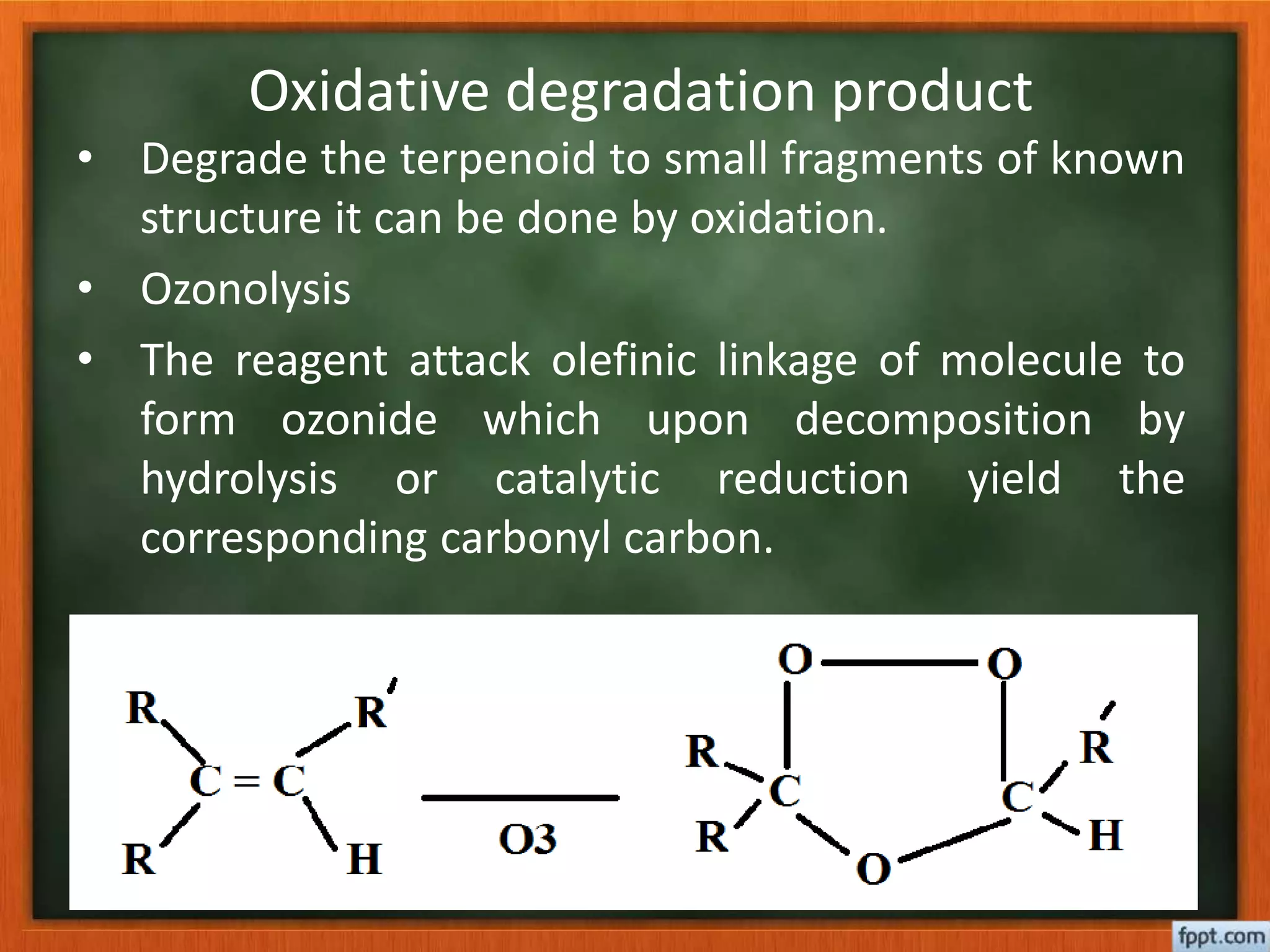
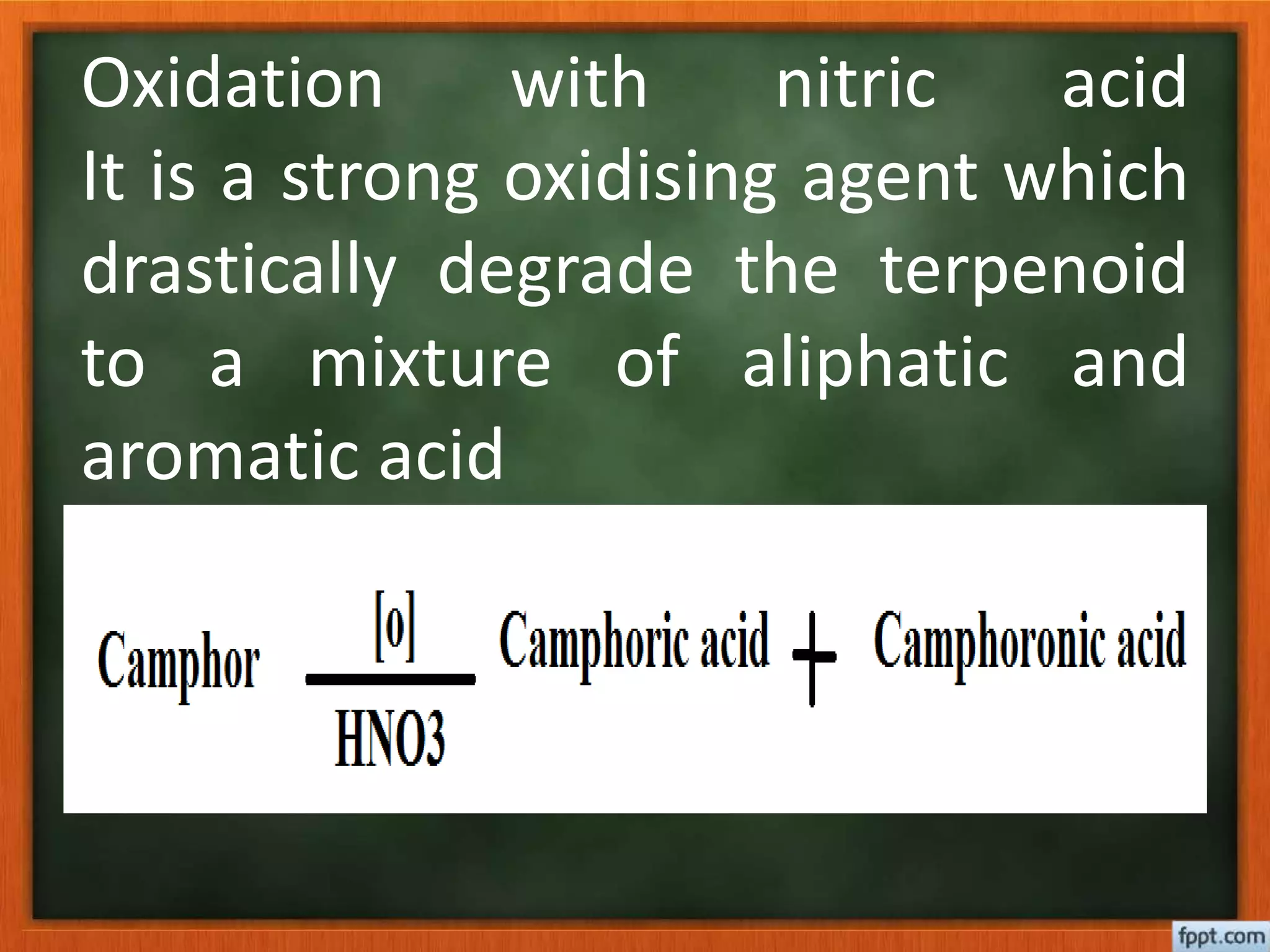
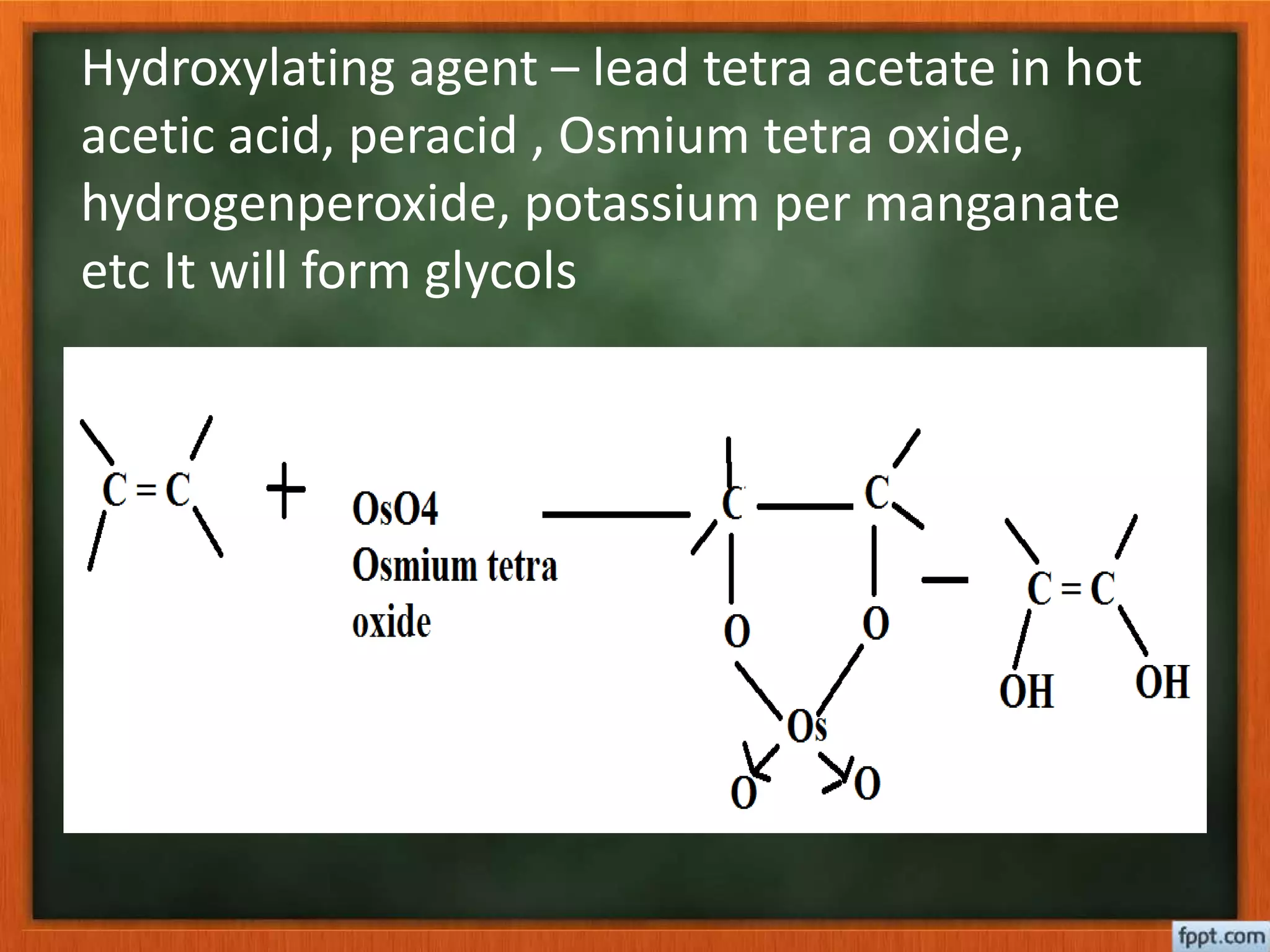
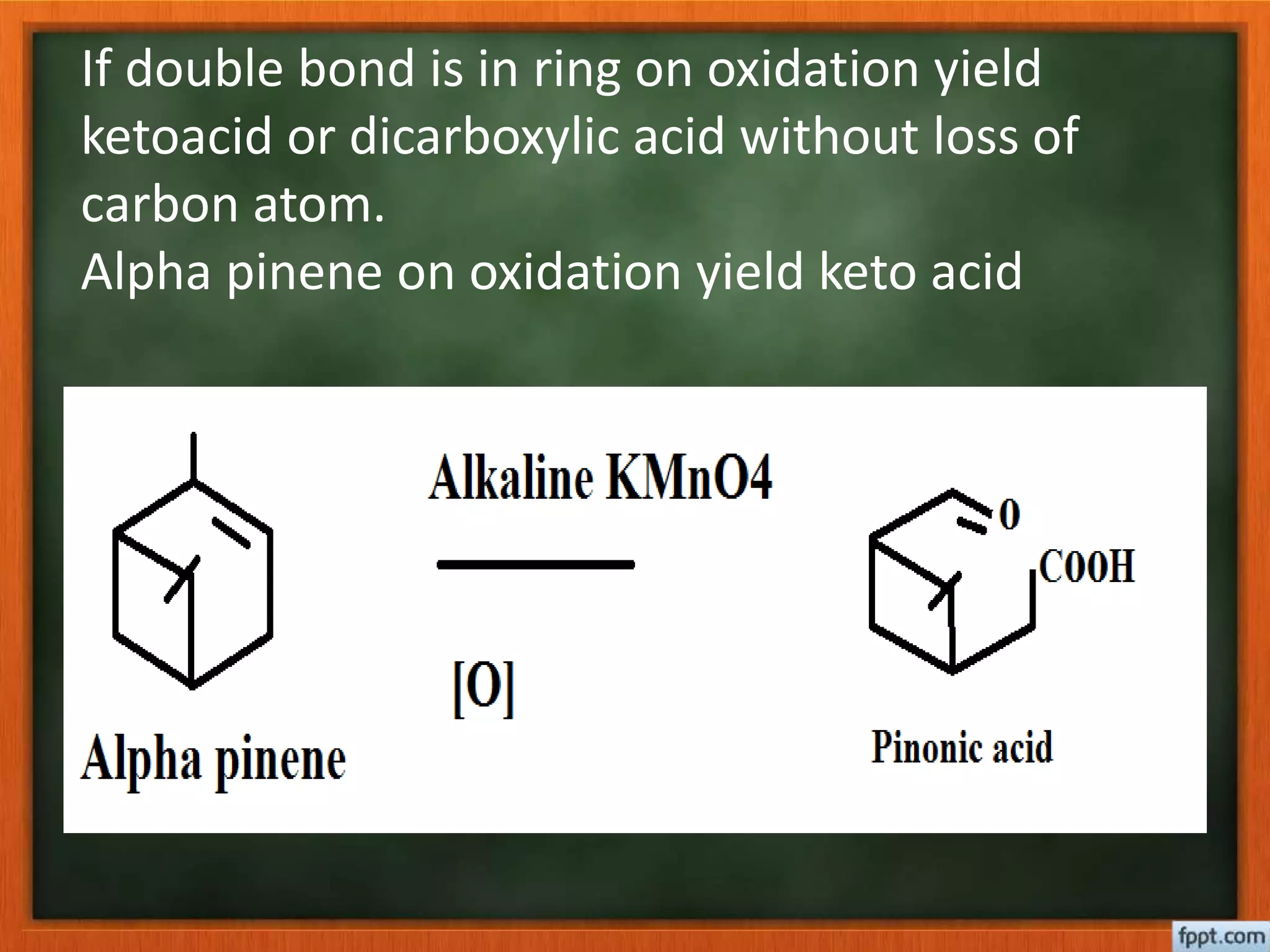
- Reactions like oxidation, ozonolysis, and degradation to break down terpenoids into fragments of known structure.
- Physical methods like UV, IR, NMR, and X-ray crystallography provide further structural information.
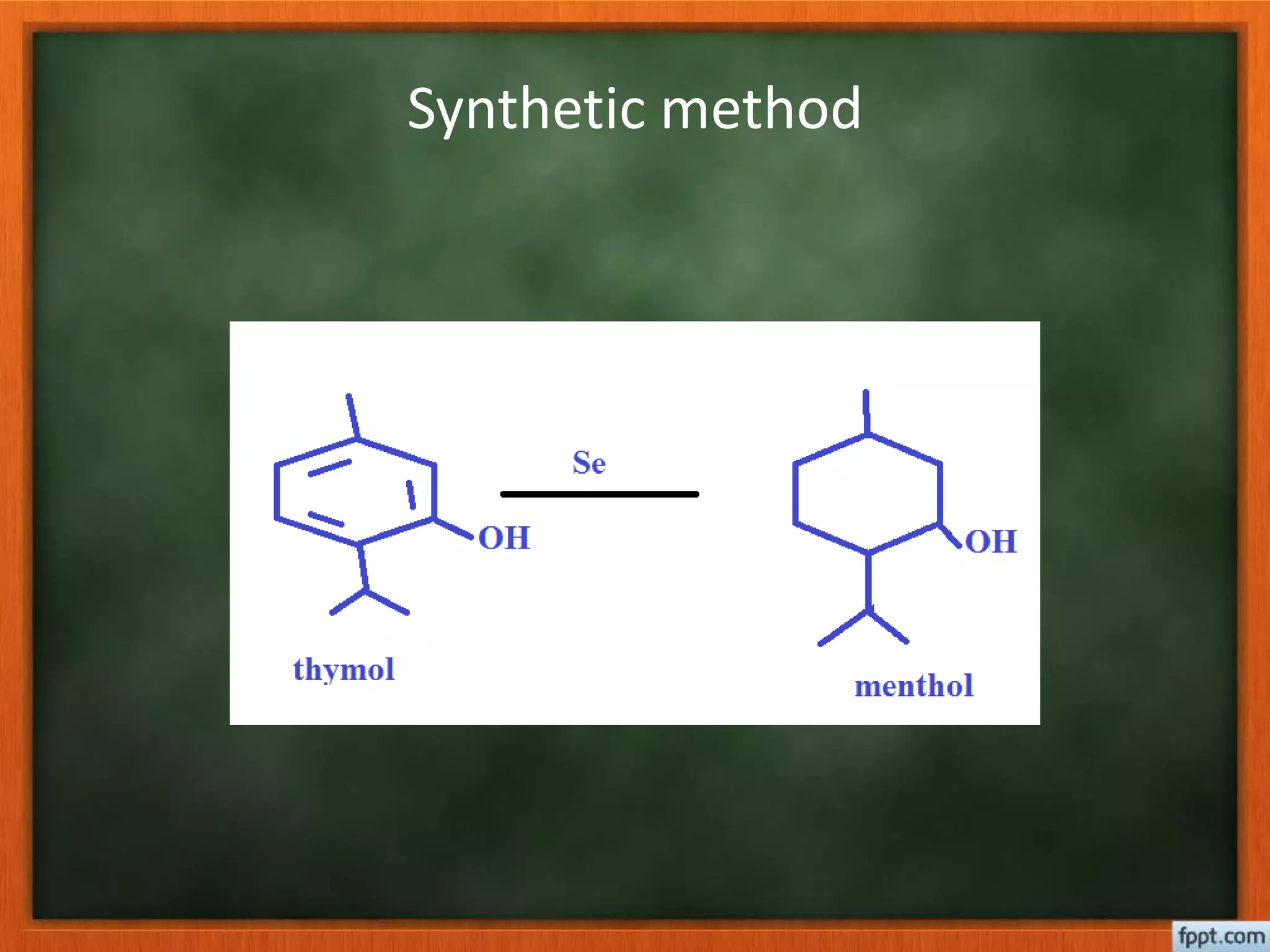
- Considering factors like number of double bonds and rings, along with molecular formula, can reveal the parent hydrocarbon skeleton. Synthetic reactions help confirm or determine unknown structures.












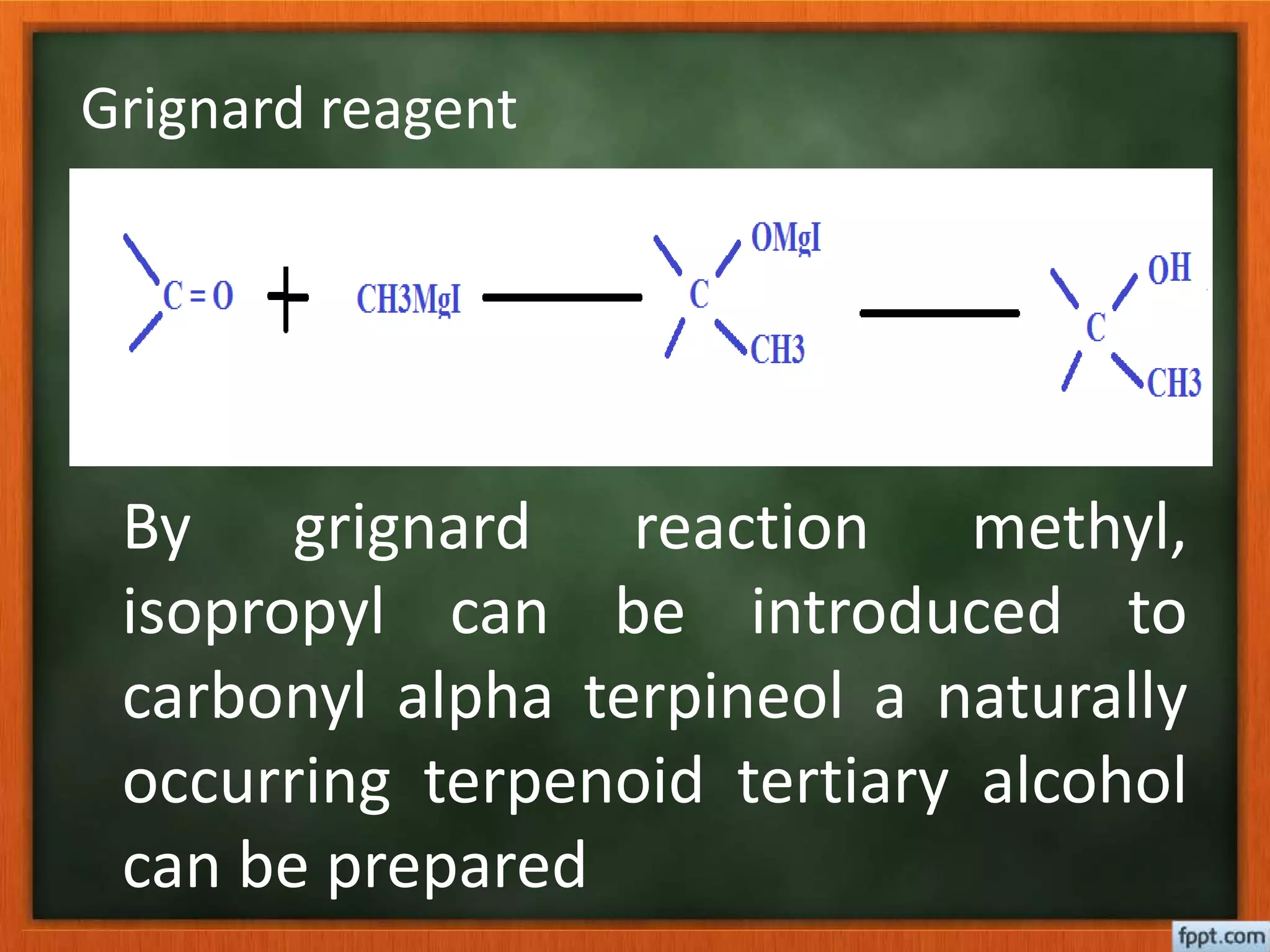
![Reformatsky reaction- alpha substituted ester
treated with carbonyl [ aldehyde / ketone /
ester ] in the presence of zinc to form beta
hydroxy ester.later which is treated with dilute
acid to yield beta hydroxy acid which may
further converted to unsaturated acid or
hydrocarbon](https://image.slidesharecdn.com/5-170223053028/75/5-Determination-of-structure-of-terpenoid-34-2048.jpg)

